Dossier na temat Anthony Fauciego/COVID-19
Ta praca została częściowo wsparta przez zbiórkę pieniędzy, w której około 330 osób przekazało fundusze na wsparcie zespołu technologicznego New Earth i Urban Global Health Alliance. Jest ona udostępniona na licencji Creative Commons CC- BY-NC-SA. Wszelkie pochodne wykorzystanie tego dossier musi być upublicznione dla dobra innych. Wszystkie dokumenty, referencje i informacje zawarte w niniejszym dokumencie, mają charakter taki jaki mają w chwili ich opublikowania. Autor nie ponosi odpowiedzialności za błędy w zapisie publicznym lub w odniesieniach do niego. W całym dokumencie, użycie terminów powszechnie przyjętych w literaturze medycznej i naukowej, nie oznacza akceptacji lub odrzucenia dogmatu, który reprezentują.
[Dossier – zbiór dokumentów dotyczących jakiejś sprawy]
SARS-CoV-2 w kontekście patentów sprzed 20 lat – dr David Martin
Wersja po angielsku – The Fauci COVID-19 Dossier
Dossier na temat Anthony Fauciego/COVID-19

Tło:
Przez ostatnie dwie dekady, moja firma – M-CAM – monitorowała możliwe naruszenia Protokołu o zakazie używania gazów duszących, trujących lub innych w czasie wojny; bakteriologicznych metod prowadzenia wojny (Protokół Genewski z 1925 roku) oraz Konwencji o zakazie prowadzenia badań, produkcji i gromadzenia zapasów broni bakteriologicznej i toksycznej oraz ich zniszczeniu (BTWC z 1972 roku). W naszej Globalnej Ocenie Technologii na lata 2003-2004: Zbrojenia Wektorowe [Global Technology Assessment: VectorWeaponization]. W M-CAM zwróciliśmy uwagę na rosnące zaangażowanie Chin, w technologię łańcuchowej reakcji polimerazy (PCR), w odniesieniu do dołączenia do światowej sceny w konstrukcji chimerycznych wektorów wirusowych. Od tego czasu, co tydzień, monitorujemy rozwój badań i wysiłki komercyjne w tej dziedzinie, włączając w to, ale nie ograniczając się, do synergii badawczej tworzącej się pomiędzy amerykańskim Centrum Kontroli i Prewencji Chorób (CDC), Narodowym Instytutem Alergii i Chorób Zakaźnych (NIAID), Uniwersytetem Północnej Karoliny w Chapel Hill (UNC), Uniwersytetem Harvarda, Uniwersytetem Emory, Uniwersytetem Vanderbilta, Uniwersytetem Tsinghua, Uniwersytetem Pensylwanii oraz wieloma innymi instytucjami badawczymi i ich komercyjnymi powiązaniami.
Grant AI23946-08 przyznany przez Narodowy Instytut Zdrowia dr Ralphowi Baricowi z Uniwersytetu Północnej Karoliny w Chapel Hill (oficjalnie sklasyfikowany jako powiązany z Narodowym Instytutem Alergii i Chorób Zakaźnych [NIAID] dr Anthony’ego Fauci przynajmniej w 2003 roku) rozpoczął prace nad syntetycznym modyfikowaniem Coronaviridae (rodzina koronawirusów) w wyraźnym celu prowadzenia ogólnych badań, wzmacniania patogenów, wykrywania, manipulowania i potencjalnych interwencji terapeutycznych, skierowanych na te same czynniki. Już 21 maja 2000 roku dr Ralph Baric i Uniwersytet Północnej Karoliny [UNC] dążyli do opatentowania krytycznych sekcji rodziny koronawirusów, dla własnych korzyści komercyjnych [U.S. Provisional Application No. 60/206,537, filed May 21, 2000]. W jednej z kilku prac, powstałych w wyniku prac sponsorowanych przez ten grant, dr R. Baric opublikował to, co według niego było pełnej długości cDNA wirusa SARS CoV, w którym wyraźnie stwierdzono, że wirus SAR CoV był oparty na złożonym segmencie DNA.
„Używając panelu przylegających cDNA, które obejmują cały genom, złożyliśmy cDNA o pełnej długości szczepu SARS-CoVUrbani i uratowaliśmy molekularnie sklonowane wirusy SARS (klon zakaźny SARS-CoV), które zawierały oczekiwane mutacje markera wstawione do klonów składowych.”- PNAS October 28, 2003 100 (22) 12995-13000; Reversegenetics with a full-lengthinfectiouscDNA of severeacute respiratory syndromecoronavirus https://www.pnas.org/content/100/22/12995
Wiosną, 19 kwietnia 2002 roku, przed pierwszym wybuchem SARS w Azji – Christopher M. Curtis, Boyd Yount i Ralph Baric złożyli wniosek o patent U.S. 7279372 na metodę wytwarzania rekombinowanego koronawirusa. W pierwszym publicznym zapisie stwierdzeń, starali się oni opatentować sposób wytwarzania „zakaźnego, defektywnego w replikacji koronawirusa”. Praca ta była wspierana przez grant NIH wspomniany powyżej i GM63228. W skrócie, amerykański Departament Zdrowia i Opieki Społecznej [HHS] był zaangażowany w finansowanie wzmacniania zakaźnej natury koronawirusów w latach 1999-2002, zanim SARS został kiedykolwiek wykryty u ludzi.
Na tym tle zauważyliśmy niezwykłe wysiłki CDC w zakresie podejmowanych starań uzyskania patentów, gdy 25 kwietnia 2003 r. starało się ono o opatentowanie koronawirusa SARS wyizolowanego od ludzi, który podobno przeniósł się na ludzi podczas epidemii SARS w Azji w latach 2002-2003. Rozdział 11, paragraf 101 Kodeksu Stanów Zjednoczonych [35 U.S.C. §101] dotyczący wynalazków podlegających opatentowaniu zabrania patentowania przyrody. Ten przepis nie powstrzymał CDC w ich wysiłkach. Ich wniosek, uaktualniony w 2007 roku, ostatecznie został wydany jako patent USA 7220852 i ograniczał każdego, kto nie był licencjonowany [upoważniony] przez ich patent, do manipulowania SARS CoV, rozwijania testów lub zestawów do pomiaru koronawirusa SARS u ludzi lub pracy z ich opatentowanym wirusem do użytku terapeutycznego. Prace związane z tym wirusem, prowadzone przez wybranych współpracowników, obejmowały: znaczne ilości inżynierii chimerycznej, badania typu uzyskiwania funkcji [gain-of-function], charakteryzację wirusa, wykrywanie, leczenie (zarówno szczepionki, jak i interwencje terapeutyczne) oraz badania nad bronią biologiczną.
Krótko mówiąc, dzięki patentowi dr Barica [USA 6593111] (roszczenia 1 i 5) oraz patentowi CDC o numerze 7220852 (roszczenie 1), żadne badania w Stanach Zjednoczonych nie mogły być prowadzone bez zezwolenia lub naruszenia tychże patentów.
Zauważyliśmy, że specjalista w dziedzinie badań nad uzyskiwaniem funkcji [gain-of-function], dr Ralph Baric, był zarówno odbiorcą milionów dolarów amerykańskich w postaci dotacji na badania od kilku agencji federalnych, jak i zasiadał w Międzynarodowym Komitecie Taksonomii Wirusów [International Committee on Taxonomy of Viruses (ICTV)] Światowej Organizacji Zdrowia oraz w Grupie Badawczej Coronaviridae (CSG).
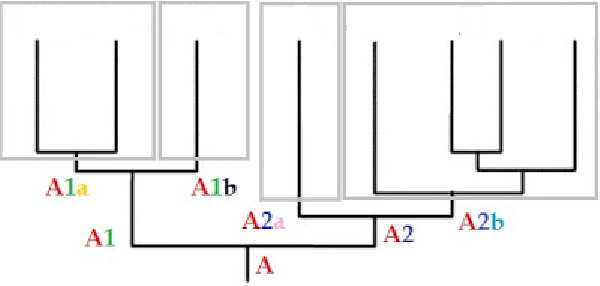
Haplogrupy [A] dzielą się na dodatkowe podgrupy (subklad) [A1a i A1b, A2a i A2b].
W tym charakterze, dr Ralph Baric był odpowiedzialny za określanie „nowości” wśród kladów różnych gatunków wirusów, ale także bezpośrednio korzystał z deklarowanych nowości – w formie nowych wniosków na finansowanie badań i związanej z nimi współpracy patentowej i handlowej. Razem z CDC, NIAID, WHO, placówkami akademickimi i podmiotami komercyjnymi (w tym Johnson & Johnson; Sanofi i ich kilkoma firmami biotechnologicznymi, posiadającymi patenty na koronawirusy; Moderna; Ridgeback; Gilead; Sherlock Biosciences; i inne), potężna grupa interesów tworzyła coś, odnośnie czego sugerowalibyśmy, że jest „wzajemnym powiązaniem/zazębianiem się dyrekcji”, zgodnie z amerykańskimi prawami antymonopolowymi.
Podmioty te były również powiązane z Globalną Radą Monitorowania Gotowości [Global Preparedness Monitoring Board [GPMB)] pod WHO, której członkowie odegrali zasadniczą rolę w finansowanym przez Open Philanthropy globalnym ćwiczeniu „przy stole” odnośnie pandemii koronawirusów o nazwie „Wydarzenie 201” w październiku 2019 roku.
To wydarzenie [Event 201], sfinansowane przez głównego inwestora w Sherlock Biosciences oraz Fundację Billa i Melindy Gatesów w mandat Globalnej Rady Monitorowania Gotowości [GPMB] na globalne ćwiczenie w sprawie gotowości na choroby układu oddechowego, które ma być zakończone do września 2020 roku, zaalarmowało nas to, bo pojawiła się wskazówka dotycząca scenariusza przyszłej „epidemii”. Spodziewaliśmy się, że taki scenariusz pojawi się w Wuhan lub Guangdong w Chinach, w północnych Włoszech, w Seattle, w Nowym Jorku lub w kombinacji tych miejsc, ponieważ prace dr Zhengli Shi i dr Barica nad odzwierzęcym przenoszeniem się koronawirusa, zidentyfikowały nakładające się mutacje koronawirusa w populacjach nietoperzy znajdujących się w tych obszarach.
Dossier ten nie jest bynajmniej wyczerpujący. Wskazuje on jednak na liczne naruszenia prawa karnego, które mogą być związane z terroryzmem COVID-19. Wszystkie materiały źródłowe są w nim cytowane. Dodatkowe szczegółowe zestawienie wszystkich osób, instytucji badawczych, fundacji, źródeł finansowania i przedsiębiorstw komercyjnych jest dostępne na życzenie.
Spis treści:
35 U.S.C. § 101 – Wynalazki Podlegające Opatentowaniu
18 U.S.C. §2339 C i nast. – Finansowanie Aktów Terroru i Spiskowanie w Celu ich Popełnienia
18 U.S.C. § 2331 §§ 802 – Akty Terroryzmu Krajowego Skutkujące Śmiercią Obywateli Amerykańskich
18 U.S.C. § 1001 – Okłamywanie Kongresu
15 U.S.C. §1-3 – Spiskowanie w Celu Prowadzenia Przestępczej Działalności Gospodarczej
15 U.S.C. §8 – Manipulacja i Alokacja Rynku
15 U.S.C. § 19 – Wzajemne Powiązania Między Dyrekcjami
35 U.S.C. §200 – 206 – Ujawnienie Interesu Rządowego/Państwowego
21 C.F.R. § 50.24 et seq., Nielegalne Badania Kliniczne
Podmioty komercyjne
Rozdział 11, paragraf 101 Kodeksu Stanów Zjednoczonych [35 U.S.C. §101]
Z opinii sędziego Clarence’a Thomasa dla większości
Paragraf 101 ustawy o patentach stanowi: „Ktokolwiek wymyśli lub odkryje nowy i użyteczny proces, maszynę, produkcję lub skład materii, lub jakiekolwiek nowe i użyteczne ich ulepszenie, może uzyskać na nie patent, z zastrzeżeniem warunków i wymagań niniejszego rozdziału.” –35 U.S.C. § 101.
Od dawna „uważamy, że przepis ten zawiera ważny domyślny wyjątek: prawa natury, zjawiska naturalne i abstrakcyjne idee nie podlegają opatentowaniu”. Mayo, 566 U.S., at , 132 S.Ct., at 1293 (wewnętrzne cytaty i nawiasy pominięte). Są one raczej „podstawowymi narzędziami pracy naukowej i technologicznej”, które leżą poza domeną ochrony patentowej. Tamże 132 S.C., 1293. Jak wyjaśnił Sąd, bez tego wyjątku, istniałoby znaczne niebezpieczeństwo, że przyznanie patentów „uwięziłoby” wykorzystanie takich narzędzi i w ten sposób „zahamowałoby przyszłe innowacje oparte na nich”. Tamże, w 132 S.Ct., na 1301. Byłoby to sprzeczne z samym sensem istnienia patentów, które istnieją po to, by promować twórczość. Diamond v. Chakrabarty, 447 U.S. 303, 309, 100 S.C. 2204, 65 L.Ed.2d 144 (1980) (Produkty natury nie są wytwarzane, a „’manifestacje… natury [są] wolne dla wszystkich ludzi i nie są zastrzeżone na wyłączność dla kogokolwiek'”). – Association for MolecularPathology v. Myriad Genetics, Inc., 569 U.S. 576 (2013)
W swojej większościowej opinii z 2013 roku Sąd Najwyższy USA wyraźnie zaznaczył, że Sąd „od dawna utrzymywał”, że natura nie podlega patentowaniu. Samo wyizolowanie DNA nie stanowi przedmiotu patentu. W swoim patencie CDC przedstawiło fałszywe i wprowadzające w błąd twierdzenia w Biurze Patentów i Znaków Towarowych Stanów Zjednoczonych, stwierdzając, że „nowo wyizolowany ludzki koronawirus został zidentyfikowany jako czynnik wywołujący SARS i nazwany SARS-CoV ” [U.S. Patent 7220852] . Na poparcie tego stwierdzenia nie przedstawiono żadnych danych „przyczynowych”.
Kiedy 25 kwietnia 2003 r. złożyli wniosek patentowy, ich pierwszym roszczeniem (i jedynym, które przetrwało do ostatecznego wydania, mimo sprzeciwu eksperta patentowego w 2006 i 2007 r.) był genom wirusa SARS CoV.
Chociaż patent ten jest wyraźnie nielegalny zgodnie z 35 U.S.C. §101, CDC nie tylko nalegało na jego przyznanie pomimo nieostatecznych i ostatecznych odrzuceń, ale także nadal płaciło opłaty za utrzymanie patentu po tym, jak decyzja Sądu Najwyższego z 2013 r. potwierdziła, że był on sprzeczny z prawem.
Ponadto CDC opatentowało wykrywanie wirusa SARS CoV przy użyciu wielu metod, w tym reakcji łańcuchowej polimerazy z odwrotną transkrypcją (RT-PCR). Dzięki temu patentowi wykluczyli oni kogokolwiek spoza ich licencjonowanych lub spiskujących interesów z legalnego angażowania się w niezależną weryfikację ich twierdzeń, że wyizolowali wirusa, że jest on czynnikiem powodującym SARS, lub że jakakolwiek terapia może być skuteczna przeciwko zgłoszonemu patogenowi.
Należy zauważyć, że wnioski patentowe CDC zostały również odrzucone w odmowach nieostatecznych i ostatecznych z powodu niekwalifikowalności na podstawie 35 U.S.C. § 102, ponieważ zostały publicznie ujawnione przed ich złożeniem. W pierwszym nieostatecznym odrzuceniu USPTO stwierdził, że genom CDC został opublikowany w czterech wpisach akcesyjnych Genbank 14, 18 i 21 kwietnia 2003 r. z identycznością od 96,8% do 99,9% identycznych sekwencji. [Źródło: USPTO Non-FinalRejection File #10822904, September 7, 2006, page 4.]
Dr Fauci wiedział i nie ujawnił dowodów na to, że patent CDC był sprzeczny z prawem, w oparciu o prace, które finansował w latach poprzedzających wybuch SARS.
Po uzyskaniu nielegalnego patentu, złożeniu petycji o unieważnienie decyzji eksperta o jego odrzuceniu, a następnie ostatecznym przyznaniu patentu, CDC okłamało opinię publiczną, twierdząc, że kontroluje patent, aby był on „publicznie dostępny”.
Rzecznik CDC Llelwyn Grant powiedział, że: „Całym celem tego patentu jest uniemożliwienie ludziom kontrolowania technologii. Robi się to po to, aby zapewnić przemysłowi i innym badaczom rozsądny dostęp do próbek.” – Źródło: 6 maj 2003, Race to Patent SARS VirusRenewsDebate]
Tragikomicznym jest fakt, że to publiczne oświadczenie obala prosty fakt, że ich własna publikacja w Genbanku w rzeczywistości uczyniła go domeną publiczną, a tym samym nie nadawała się do opatentowania. Fakt ten, potwierdzony przez inspektorów patentowych, został unieważniony przez CDC w ramach płatnej akwizycji mającej na celu obejście prawa.
Chociaż nie jest to objęte 35 U.S.C. §101, nadużycie prawa patentowego przez dr Anthony Fauciego jest szczegółowo opisany poniżej. Na uwagę zasługuje jednak jego rozmyślne i zwodnicze użycie terminu „szczepionka” w patentach i publicznych wypowiedziach, w celu wypaczenia znaczenia tego terminu dla manipulacji opinią publiczną.
W sprawie Jacobson v. Mass z 1905 roku sąd jasno stwierdził, że aby szczepionka była obowiązkowa, wymagana jest KORZYŚĆ PUBLICZNA. Ani Pfizer, ani Moderna nie udowodniły zakłócenia w transmisji [zaraźliwości] wirusa. W sprawie Jacobson v. Massachusetts, 197 U.S. 11 (1905), sąd stwierdził, że kontekst ich opinii opiera się na następującej zasadzie:
„Ten sąd więcej niż raz uznał to za fundamentalną zasadę, że 'osoby i własność podlegają wszelkiego rodzaju ograniczeniom i obciążeniom w celu zabezpieczenia ogólnego komfortu, zdrowia i dobrobytu państwa…”.
W testach „rzekomej szczepionki” Moderna i Pfizer wyraźnie przyznali, że ich technologia terapii genowej nie ma żadnego wpływu na infekcję wirusową lub przenoszenie wirusa, a jedynie przekazuje biorcy zdolność do endogennej produkcji białka kolcowego S1 poprzez wprowadzenie syntetycznej sekwencji mRNA. Dlatego podstawa statutu Massachusetts i ustalenia Sądu Najwyższego jest w tym przypadku nieistotna.
Co więcej, Urząd Patentów i Znaków Towarowych Stanów Zjednoczonych [USPTO], podczas procesu REJESTRACJI szczepionki przeciwko HIV, który to wniosek Anthony Fauci złożył, otrzymał następującą odpowiedź na poparcie odrzucenia jego fałszywego „wynalazku”:

Wniosek/numer kontrolny: 09/869,003 Strona 5
Te argumenty są przekonujące w zakresie, w jakim antygenowy peptyd stymuluje odpowiedź immunologiczną, która może wytworzyć przeciwciała wiążące się z określonym peptydem lub białkiem, ale nie są przekonujące w odniesieniu do szczepionki. Odpowiedź immunologiczna wywołana przez szczepionkę musi być czymś więcej niż tylko pewną reakcją immunologiczną, ale musi mieć charakter ochronny. Jak zauważono w poprzedniej skardze do Urzędu, w sztuce [medycznej] termin „szczepionka” oznacza środek, który zapobiega infekcji. Wnioskodawca nie wykazał, że zgłoszona szczepionka spełnia nawet niższy standard określony w specyfikacji, a tym bardziej standardową definicję sztuki, aby być skuteczną w tym zakresie.
Dlatego roszczenia 5, 7 i 9 nie są skuteczne jako szczepionka przeciw HIV-1, a zatem nie mają patentowej użyteczności.
18 U.S.C. §2339 C i nast. – Finansowanie Aktów Terroru i Spiskowanie w Celu ich Popełnienia
Pośrednio, bezprawnie i umyślnie dostarcza lub gromadzi fundusze z zamiarem ich wykorzystania lub ze świadomością, że fundusze te mają zostać wykorzystane, w całości lub w części, w celu przeprowadzenia:
(A) czynu, który stanowi przestępstwo w ramach traktatu określonego w podsekcji (e)(7), wdrożonego przez Stany Zjednoczone, lub
(B) jakiegokolwiek innego czynu mającego spowodować śmierć lub poważne uszkodzenie ciała osoby cywilnej lub każdej innej osoby nie biorącej czynnego udziału w działaniach wojennych w sytuacji konfliktu zbrojnego, jeżeli celem takiego czynu, ze względu na jego charakter lub kontekst, jest zastraszenie ludności lub zmuszenie rządu lub organizacji międzynarodowej do podjęcia lub zaniechania działania….
Nie później niż 11 kwietnia 2005 r. dr Anthony Fauci publicznie uznał związek SARS z potencjałem bioterrorystycznym. Wykorzystując strach przed bioterroryzmem wywołanym przez wąglik w 2001 roku, publicznie świętował gospodarcze dobrodziejstwo, jakie ludzie od zajmowania się terroryzmem krajowym skierowali na jego budżet. W szczególności stwierdził, że NIAID aktywnie finansuje badania nad mikromacierzą DNA „SARS Chip” do szybkiego wykrywania SARS (coś, co nie zostało udostępnione podczas obecnej „pandemii”) i dwoma kandydatami na szczepionki skoncentrowane na białku kolcowych SARS-CoV.
Emerging Infectious Diseases: a 10-Year Perspective from the National Institute of Allergy and Infectious Diseases
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3320336/
Pod kierownictwem trzech zatrudnionych przez siebie chińskich badaczy – Zhi-yong Yanga, Wing-pui Konga i YueHuanga – Fauci miał do 2004 roku co najmniej jedną szczepionkę DNA w trakcie badań na zwierzętach.
A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7095382/
Zespół ten, wchodzący w skład Centrum Badań nad Szczepionkami przy NIAID, koncentrował się przede wszystkim na opracowywaniu szczepionek przeciwko HIV, ale jego zadaniem było również zidentyfikowanie kandydatów na szczepionkę przeciwko SARS. We współpracy z Sanofi, ScrippsInstitute, Harvardem, MIT i NIH, decyzja dr Fauci o jednostronnym promowaniu szczepionek jako podstawowej interwencji w przypadku kilku wyznaczonych „chorób zakaźnych” uniemożliwiła zastosowanie sprawdzonych terapii u chorych i umierających.
Chloroquine is a potent inhibitor of SARS coronavirus infection and spread
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1232869/
Dr Peter McCullough o blokowaniu metod leczenia Covid-19
Leczenie chorób wirusowych: czy prawdę ukrywano przez dziesięciolecia? – dr Lee D. Merritt
CDC i NIAID pod kierownictwem Anthony’ego Fauci weszły w wymianę handlową między państwami (w tym, ale nie tylko, współpraca z EcoHealth Alliance Inc.) i z obcymi narodami (w szczególności z Instytutem Wirusologii w Wuhan i Chińską Akademią Nauk) poprzez grant z 2014 roku, o oznaczeniu R01AI110964 Narodowego Instytutu Zdrowia [NIH], w celu wykorzystania swoich praw patentowych. Wiadomo było, że badania te dotyczyły białek powierzchniowych koronawirusów, które miały zdolność do bezpośredniego zakażania ludzkich układów oddechowych. Mimo rażącego naruszenia moratorium NIH na badania typu uzyskiwania funkcji [gain of function], NIAID i dr Ralph Baric kontynuowali prace nad chimerycznymi składnikami koronawirusów, specjalnie w celu wzmocnienia patogenności materiału biologicznego.
Do października 2013 roku, w ramach prac finansowanych przez NIAID w Chinach, Instytut Wirusologii w Wuhan opisał białko kolcowe S1 1 koronawirusa. W prace te zaangażowane były NIAID, USAID oraz Peter Daszak, szef EcoHealth Alliance. Praca ta, finansowana w ramach grantu R01AI079231, była kluczowa w izolowaniu i manipulowaniu fragmentami wirusów wybranych z różnych miejsc w całych Chinach, które zawierały wysokie ryzyko poważnej reakcji u ludzi.
Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor
https://pubmed.ncbi.nlm.nih.gov/24172901/
Do marca 2015 r. wiadomo było, że zarówno wirulencja białka kolcowe S1, jak i receptor ACE2 stanowią znaczne zagrożenie dla zdrowia ludzkiego. NIAID, EcoHealth Alliance i liczni badacze ubolewali nad faktem, że opinia publiczna nie była wystarczająco zaniepokojona koronawirusem, aby odpowiednio finansować ich pożądane badania.
National Academies Press (US); 2016 Feb 12. 6, Developing MCMs for Coronaviruses https://www.ncbi.nlm.nih.gov/books/NBK349040/
Dr Peter Daszak z EcoHealth Alliance zaproponował następującą ocenę sytuacji:
„Daszak powtórzył, że dopóki kryzys związany z chorobami zakaźnymi nie stanie się bardzo realny i nie przekroczy progu sytuacji kryzysowej, jest często w dużym stopniu ignorowany. Aby utrzymać bazę finansową po zakończeniu kryzysu, musimy zwiększyć świadomość opinii publicznej odnośnie konieczności opracowania medycznych środków zapobiegawczych, takich jak powszechny program uniwersalnych szczepień przeciwko koronawirusom. Kluczowym elementem napędzającym ten proces są media, a gospodarka szybko podchwyci ten trend. Musimy wykorzystać ten szum medialny i zaadresować prawdziwe problemy. Inwestorzy się pojawią, gdy tylko wyczują potencjalne zyski z finalizacji tego procesu, stwierdził Daszak.” – Źródło: Rapid Medical Countermeasure Response to Infectious Diseases: Enabling Sustainable Capabilities Through Ongoing Public- and Private-Sector Partnerships: Workshop Summary.
Ekonomia będzie podążać za trendem/szumem.
CDC i NIAID weszły w wymianę handlową między Stanami (w tym, ale nie ograniczając się do pracy z Uniwersytetem Północnej Karoliny w Chapel Hill [UNC]) i z zagranicą (w szczególności, Instytutem Wirusologii w Wuhan i Chińską Akademią Nauk reprezentowanych przez Shi Zheng-Li) poprzez U19AI109761 (Ralph S. Baric), U19AI107810 (Ralph S. Baric), i dofinansowanie z Narodowej Fundacji Nauk Przyrodniczych w Chinach 81290341 (Zheng-Li Shi) i wsp. 2015-2016. Projekty te miały miejsce w czasie, gdy wykonywane prace były zakazane przez amerykańskie Narodowy Instytut Zdrowia [NIH].
Wytworzony w laboratorium koronawirus wywołał debatę – Jef Akst [2015]
Opinia publiczna została wyraźnie poinformowana o niebezpieczeństwach, jakie niosą ze sobą badania finansowane przez NIAID w 2015 i 2016 roku, kiedy materiał z Instytutu Wirusologii w Wuhan był obrabiany w placówce Uniwersytetu Północnej Karoliny [UNC], czyli laboratorium dr Ralpha Barica.
„Jedynym skutkiem tej pracy, jest stworzenie w laboratorium nowego, nienaturalnego zagrożenia” – zgadza się Richard Ebright, biolog molekularny i ekspert ds. obrony biologicznej na Rutgers University w Piscataway, w stanie New Jersey. Zarówno Ebright jak i Wain-Hobson są długoletnimi krytykami badań typu gain-of-function [uzyskiwania funkcji].
W swoim artykule autorzy badania przyznają również, że fundatorzy mogą/powinni się dobrze zastanowić o dopuszczeniu takich eksperymentów w przyszłości. „Panele naukowe mogą uznać, że podobne badania, nad budową wirusów chimerycznych, opartych na krążących szczepach, są zbyt ryzykowne, aby je prowadzić” – piszą, dodając, że potrzebna jest dyskusja na temat tego, „czy tego typu badania nad wirusami chimerycznymi uzasadniają dalsze badania w porównaniu z nieodłącznym ryzykiem”.
Ale dr Baric i inni twierdzą, że badania przyniosły korzyści. Wyniki badań „przesuwają tego wirusa z kandydata na nowo pojawiający się patogen do wyraźnego i obecnego zagrożenia”, mówi dr Peter Daszak, który był współautorem pracy z 2013 roku. Peter Daszak jest prezesem EcoHealth Alliance, międzynarodowej sieci naukowców z siedzibą w Nowym Jorku, która pobiera próbki wirusów od zwierząt i ludzi w miejscach występowania nowych chorób na całym świecie.
Dr Peter Daszak zgadza się, że badania testujące hybrydowe wirusy w ludzkich hodowlach komórkowych i modelach zwierzęcych mają ograniczony zakres, jeśli chodzi o to, co mogą powiedzieć o zagrożeniu stwarzanym przez dzikiego wirusa. Twierdzi on jednak, że mogą one pomóc wskazać, które patogeny powinny być traktowane priorytetowo w dalszych badaniach.” – Nature, 12 listopad 2015, Engineered bat virus stirs debate over risky research https://www.nature.com/articles/nature.2015.18787
Anthony Fauci: „Korzyści ze zgromadzonych danych przeważają nad ryzykiem związanym z pandemią” – dr Chris Martenson
EcoHealth Alliance dr Petera Daszaka ukryło prawie 40 milionów dolarów z Pentagonu i zmilitaryzowało naukę o pandemii – Sam Husseini
Wiedząc, że amerykański Departament Zdrowia i Usług Społecznych [HHS] (poprzez CDC, NIH, NIAID oraz finansowane przez nich laboratoria i partnerów komercyjnych) miał patenty na każdy proponowany element medycznych środków zaradczych i ich finansowanie, dr Fauci, dr Gao (Chińskie CDC) i dr Elias (Fundacja Billa i Melindy Gates) spiskowali, aby popełnić akty terroru na globalnej populacji – w tym obywatelach Stanów Zjednoczonych – kiedy we wrześniu 2019 r. opublikowali następujące informacje w corocznym raporcie na temat globalnej gotowości na sytuacje kryzysowe związane ze zdrowiem:
„Kraje, darczyńcy i instytucje wielostronne muszą być przygotowane na najgorsze.
Szybko rozprzestrzeniająca się pandemia spowodowana śmiertelnym patogenem oddechowym (czy to naturalnie pojawiającym się, czy też przypadkowo lub celowo uwolnionym), stawia dodatkowe wymagania dotyczące gotowości. Darczyńcy i instytucje wielostronne muszą zapewnić odpowiednie inwestycje w rozwój innowacyjnych szczepionek i terapii, zdolności produkcyjnych, leków antywirusowych o szerokim spektrum działania oraz odpowiednich interwencji niefarmakologicznych. Wszystkie kraje muszą opracować system natychmiastowego udostępniania sekwencji genomu każdego nowego czynnika chorobotwórczego do celów zdrowia publicznego, a także środki umożliwiające wymianę ograniczonych medycznych środków zaradczych między krajami.
Wskaźnik(i) postępu do września 2020 roku:
– Darczyńcy i kraje zobowiązują się i określają harmonogramy: finansowania i opracowania uniwersalnej szczepionki przeciwko grypie, leków antywirusowych o szerokim spektrum działania i terapii celowanych. WHO i jej państwa członkowskie opracowują opcje standardowych procedur i harmonogramów udostępniania danych dotyczących sekwencji, próbek i medycznych środków zaradczych dla patogenów innych niż grypa.
– Darczyńcy, kraje i instytucje wielostronne opracowują wieloletni plan i podejście do wzmocnienia zdolności badawczych w zakresie badań i rozwoju, przed i w trakcie epidemii.
– WHO, Fundusz Narodów Zjednoczonych na rzecz Dzieci, Międzynarodowa Federacja Stowarzyszeń Czerwonego Krzyża i Czerwonego Półksiężyca, partnerzy akademiccy i inni partnerzy określają strategie zwiększania możliwości i integracji podejść i badaczy z dziedziny nauk społecznych w całym kontinuum gotowości i reagowania ” – str. 8, Annual report on global preparedness for health emergencies
Jakby na potwierdzenie użyteczności żądania z września 2019 r. o „finansowanie i rozwój” szczepionki oraz fortunnego rzekomego wybuchu SARS CoV-2 w grudniu 2019 r., dr Fauci zaczął napawać się, że jego losy w zakresie dodatkowego finansowania prawdopodobnie zmieniają się na lepsze. W wywiadzie w STAT z 10 lutego 2020 roku, został on zacytowany w następujący sposób:
„”Pojawienie się nowego wirusa zmieni tę liczbę, prawdopodobnie znacznie”, powiedział Fauci. „Nie wiem, jak dużo to będzie. Ale myślę, że to będzie generować bardziej trwałe zainteresowanie koronawirusami, ponieważ jest bardzo jasne, że koronawirusy mogą dokonywać naprawdę interesujących rzeczy.”” – Fluctuating funding and flagging interest hurt coronavirus research, leaving crucial knowledge gaps
18 U.S.C. § 2331 §§ 802 – Akty Terroryzmu Krajowego Skutkujące Śmiercią Obywateli Amerykańskich
Sekcja 802 amerykańskiej ustawy PATRIOT Act (Pub. L. No. 107-52) rozszerzyła definicję terroryzmu, aby objąć „krajowy,” w przeciwieństwie do międzynarodowego, terroryzm. Osoba angażuje się w terroryzm krajowy, jeśli dokonuje czynu „niebezpiecznego dla życia ludzkiego”, który jest pogwałceniem praw karnych stanu lub Stanów Zjednoczonych, jeśli czyn ten wydaje się być zamierzony, aby: (i) zastraszyć lub wymusić [coś] na ludności cywilnej; (ii) wywrzeć wpływ na politykę rządu poprzez zastraszenie lub wymuszenie;
Dr Anthony Fauci zastraszał i zmuszał ludność cywilną oraz próbował wpłynąć na politykę rządu poprzez zastraszenie i przymus.
Bez żadnego potwierdzenia, dr Anthony Fauci promował symulację komputerową profesora Neila Fergusona, wywodząc z niej twierdzenia, że:
„Świat stoi w obliczu najpoważniejszego kryzysu zdrowia publicznego od pokoleń. Tutaj przedstawiamy konkretne szacunki skali zagrożenia, przed jakim stoją obecnie kraje.”
„Używamy najnowszych szacunków dotkliwości, aby pokazać, że strategie polityczne, których celem jest złagodzenie epidemii, mogą zmniejszyć liczbę zgonów o połowę i ograniczyć szczyt zapotrzebowania na opiekę zdrowotną o dwie trzecie, ale to nie wystarczy, aby zapobiec przeciążeniu systemów opieki zdrowotnej. Aby ograniczyć przenoszenie choroby do niskiego poziomu, konieczne będą zatem bardziej intensywne i społecznie destrukcyjne interwencje. Jest prawdopodobnym, że takie środki – przede wszystkim dystans społeczny na dużą skalę – będą musiały być stosowane przez wiele miesięcy, być może aż do momentu, gdy dostępna będzie szczepionka.” – Imperial College London, 17 marca 2020, COVID-19: Imperial researchers model likely impact of public healthmeasures
Przegląd kodu źródłowego z modelu Fergusona
COVID – dlaczego terminologia ma znaczenie? – dr Malcolm Kendrick
Plusy i minusy środków zaradczych przeciw pandemii grypy – dr Thomas Inglesby, prof. Jennifer Nuzzo, prof. Tara O’Toole i prof. Donald Henderson [listopad 2006]
Lockdown jest ponad 10 razy bardziej śmiertelny niż sama pandemia
Informując prezydenta, że aż 2,2 miliona zgonów może być skutkiem patogenu, który nie został jeszcze wyizolowany i którego nie można było zmierzyć z żadną dokładnością, dr Fauci zastraszył i zmusił ludność oraz rząd do lekkomyślnych, niesprawdzonych i szkodliwych działań, powodujących nieodwracalne szkody dla życia i środków do życia.
Ani Imperial College, ani „niezależny” Institute for HealthMetrics and Evaluation [Instytut Mierzenia i Oceny Zdrowia] (finansowany głównie przez Fundację Billa i Melindy Gatesów)nie miały żadnych dowodów na sukces w szacowaniu wcześniejszych obciążeń spowodowanych koronawirusem, ale bez konsultacji czy wzajemnej weryfikacji dr Fauci przyjął ich przerażające szacunki za podstawę interwencji, które są wyraźnie sprzeczne z zaleceniami medycznymi.
– Nałożenie dystansu społecznego było oparte na symulacji komputerowej i modelach środowiskowych, które nie zawierały żadnych dowodów na przenoszenie chorób.
– Narzucenie noszenia masek na twarz było bezpośrednio sprzeczne z dowodami z kontrolowanych badań klinicznych i z pisemną polityką w czasopiśmie Amerykańskiego Stowarzyszenia Medycznego [AMA].
„Maski na twarz nie powinny być noszone przez zdrowe osoby w celu ochrony przed nabyciem infekcji dróg oddechowych, ponieważ nie ma dowodów sugerujących, że maski na twarz noszone przez zdrowe osoby są skuteczne w zapobieganiu chorobie.” – JAMA. 2020;323(15):1517-1518; Medical Masks
– Zarówno w symulacjach Imperial College, jak i IHME, kwarantanny były modelowane dla osób chorych, a nie zdrowych.
Geneza idei zamykania kraju [Lockdown] sięga 2006 roku – Jeffrey A. Tucker [American Institute for Economic Research]
Maseczki nie działają: Przegląd literatury naukowej w kontekście zasadności polityki społecznej wobec COVID-19 – dr Denis Rancourt
Naleganie na szczepionki, przy jednoczesnym blokowaniu awaryjnego stosowania sprawdzonych interwencji farmaceutycznych, mogło przyczynić się do śmierci wielu pacjentów i zdrowych osób.
Wykorzystując władzę NIAID podczas domniemanej pandemii, dr Anthony Fauci aktywnie tłumił sprawdzone medyczne środki zaradcze, stosowane i potwierdzone w postępowaniach naukowych, które oferowały alternatywę dla produktów finansowanych przez jego spiskujące podmioty, którym zapewnił bezpośrednie finansowanie i dla których miał otrzymywać korzyści materialne i niematerialne.
Alan Jones: Biurokraci zaprzeczają, że hydroksychlorochina zmniejsza śmiertelność
Koronawirus: Jak sobie z nim poradzić? – Prof. Didier Raoult [Chlorochina]
Hydroksychlorochina – czy działa na koronawirusa? – Dr Vladimir Zelenko
Wywiad z dr Stellą Immanuel o Hydroksychlorochinie [30 lipiec 2020]
COVID-19 – Dr Richard Bartlett: Strategia oparta na leku Budezonid
18 U.S.C. § 1001 – Okłamywanie Kongresu
(a)O ile niniejsza sekcja nie stanowi inaczej, każdy, kto w jakiejkolwiek sprawie podlegającej jurysdykcji władzy wykonawczej, ustawodawczej lub sądowniczej Rządu Stanów Zjednoczonych, świadomie i umyślnie-
(1) fałszuje, ukrywa lub tuszuje za pomocą jakiejkolwiek sztuczki, schematu lub urządzenia istotny fakt;
(2) składa jakiekolwiek materialnie fałszywe, fikcyjne lub oszukańcze oświadczenie lub reprezentację; lub
(3) sporządza lub wykorzystuje fałszywe pismo lub dokument, wiedząc, że zawiera on fałszywe, fikcyjne lub oszukańcze oświadczenie lub wpis; podlega grzywnie na mocy niniejszego paragrafu, karze pozbawienia wolności na okres nie dłuższy niż 5 lat lub, jeśli przestępstwo wiąże się z międzynarodowym lub krajowym terroryzmem (zgodnie z definicją w sekcji 2331), karze pozbawienia wolności na okres nie dłuższy niż 8 lat, lub obu tym karom. Jeżeli sprawa dotyczy przestępstwa z rozdziału 109A, 109B, 110 lub 117, lub sekcji 1591, wówczas kara pozbawienia wolności nałożona na mocy niniejszej sekcji nie przekracza 8 lat.
W dniu 22 października 2020 r. Biuro Odpowiedzialności Rządu Stanów Zjednoczonych (GAO) opublikowało raport zatytułowany:
W dokumencie tym autorzy poinformowali, że Narodowy Instytut Zdrowia (NIH) otrzymał „do 2 miliardów dolarów w tantiemach z tytułu wkładu w 34 leki sprzedane w latach 1991-2019”.
Pobieżny przegląd raportu NIH Office of Technology Transfer dotyczącego aktywnych licencji wydaje się sprzeczny z raportem GAO w odniesieniu do kilku istotnych faktów. W raporcie GAO wyraźnie brakuje ponad 30 patentów związanych z aktywnymi związkami generującymi miliardy dolarów przychodu. Dlaczego GAO i NIH nie mogły dojść do porozumienia w sprawie czegoś tak prostego, jak leki generujące dochód dla NIH?
Od czasu uchwalenia ustawy Bayh Dole Act (Pub. L. 96-517, 12 grudnia 1980 r.), badania finansowane z funduszy federalnych były ekonomiczną bonanzą dla amerykańskich uniwersytetów, agencji federalnych i ich wybranych patronów. W pierwszej dekadzie po wprowadzeniu ustawy Bayh-Dole, fundusze NIH podwoiły się z 3,4 miliarda dolarów do 7,1 miliarda dolarów. Dekadę później- podwoiły się ponownie- osiągając 15,6 miliarda dolarów. W następstwie września 2001 roku, Narodowy Instytut Alergii i Chorób Zakaźnych (NIAID) odnotował bezpośredni wzrost swojego budżetu o ponad 300%, bez uwzględnienia funduszy DARPA, które od 2005 roku wynosiły aż 1,7 miliarda dolarów rocznie. W 2020 roku budżet NIH wynosił ponad 41 miliardów dolarów.
Co się stało z 763 miliardami dolarów z funduszy podatników przeznaczonych na uczynienie Ameryki zdrowszą, odkąd wynalazcy są zachęcani do komercyjnego działania? Kto się wzbogacił?
Odpowiedź, niestety, jest taka, że skrupulatne rozliczenia nie są prowadzone, aby móc odpowiedzieć na te pytania.
Narodowy Instytut Zdrowia [NIH] jest wymienionym z nazwy właścicielem co najmniej 138 patentów od 1980 roku.
Amerykański Departament Zdrowia i Opieki Społecznej [HHS] jest wymienionym z nazwy właścicielem co najmniej 2600 patentów.
Dotacje lub współpraca z NIAID zaowocowały 2655 patentami i wnioskami patentowymi, z których tylko 95 zawiera przypisanie do Departamentu Zdrowia i Usług Społecznych jako właściciela. Większość z tych patentów jest przypisana do uniwersytetów, co sprawia, że ostateczni beneficjenci komercyjni są całkowicie nieprzejrzyści. Jednym z największych posiadaczy jest SIGA Technologies (NASDAQ: SIGA), która, choć publicznie informuje o bliskich związkach z NIAID, nie jest wymieniona w raporcie NIH GAO. Dyrektor generalny SIGA, dr Phillip L. Gomez spędził 9 lat w NIAID, rozwijając program szczepionek przeciwko HIV, SARS, Ebola, Wirusa Zachodniego Nilu i grypy, zanim przeszedł do przedsięwzięć komercyjnych. Chociaż ich technologia wyraźnie wywodzi się z osiągnieć naukowych do których przyczynił się NIAID, firma zgłasza przychody z NIAID, ale nie ma żadnych opłat licencyjnych ani płatności komercyjnych na rzecz NIH lub któregokolwiek z jego programów.
Dyrektor NIAID, dr Anthony Fauci, jest wymieniony jako wynalazca w 8 przyznanych patentach amerykańskich. Żaden z nich nie został zgłoszony w raportach NIAID, NIH lub GAO dotyczących aktywnego licencjonowania, pomimo faktu, że dr Fauci został podobno zmuszony do otrzymania zapłaty za swój „wynalazek” w postaci Interleukiny-2 – płatności, które podobno przekazał nienazwanej organizacji charytatywnej.
Z 21 patentów wymienionych w pomarańczowej księdze amerykańskiej Agencji ds. Żywności i Leków (FDA), wymienionych w raporcie GAO, żaden z patentów dr Anthony’ego Fauci nie jest wymieniony. Co więcej, żaden z patentów NIAID nie jest wymieniony pomimo wyraźnych dowodów na to, że Gilead Sciences i Janssen Pharmaceuticals (oddział Johnson & Johnson) wygenerowały ponad 2 miliardy dolarów rocznie ze sprzedaży, która była bezpośrednim rezultatem osiągnięć naukowych finansowanych przez NIAID. W raporcie GAO brakuje 2 patentów na Velclade, który od kilku lat generuje sprzedaż przekraczającą 2,18 miliarda dolarów rocznie. Żaden z patentów dla Yescarta nie jest wymieniony w raporcie GAO. Żaden z patentów na Lumoxiti nie jest wymieniony w raporcie GAO. Żaden z patentów na lek Kepivance nie został wymieniony w raporcie GAO. Z naruszeniem 37 USC §410.10 i 35 USC §202(a), ponad 13 z 21 patentów w raporcie GAO nie ujawnia interesów rządu, mimo że są one bezpośrednim wynikiem finansowania przez NIH.
Odporność i bezkarność: korupcja w relacjach Państwo-Farmacja – dr PaddyRawlinson
Prawda o firmach farmaceutycznych. Jak nas oszukują i co z tym robić – dr MarciaAngell
Własny dorobek patentowy dr Anthony’ego Fauci:
Patent US 6190656 i 6548055 Wzmocnienie immunologiczne z przerywaną terapią interleukiną-2
Metoda aktywacji układu odpornościowego ssaków obejmuje serię podań IL-2, co odbywa się z przerwami przez dłuższy okres czasu. Każde podanie IL-2 jest wystarczające, aby umożliwić wzrost i szczyt spontanicznej syntezy DNA w komórkach krwi obwodowej lub węzłów chłonnych pacjenta, a każde następne podanie następuje po poprzednim podaniu w serii przez okres czasu, który jest wystarczający, aby umożliwić ekspresję receptora IL-2 w krwi obwodowej lub węzłach chłonnych pacjenta, aby wzrosnąć, osiągnąć szczyt, a następnie zmniejszyć się do 50% wartości szczytowej. Ta przerywana terapia IL-2 może być łączona z inną terapią, która jest ukierunkowana na określony stan chorobowy, taką jak terapia antyretrowirusowa- obejmująca, na przykład, podawanie AZT, ddI lub interferonu alfa. Ponadto, podawanie IL-2 może być stosowane w celu ułatwienia transdukcji in situ limfocytów T w kontekście terapii genowej. W tym podejściu komórki są najpierw aktywowane in vivo przez wspomnianą terapię IL-2, a następnie transdukcja jest dokonywana przez dostarczenie genetycznie zmodyfikowanego wektora retrowirusowego bezpośrednio do pacjenta.
Niniejsze zgłoszenie jest kontynuacją amerykańskiego zgłoszenia patentowego nr ser. 08/487075, złożonego 7 czerwca 1995 r., obecnie zaniechanego, które jest kontynuacją w części amerykańskiego zgłoszenia patentowego nr ser. 08/063315, złożonego 19 maja 1993 r., obecnie wydanego jako patent US 5419900, oraz zgłoszenie patentowe USNr ser. 08/452440, złożone 26 maja 1995 r., obecnie wydane jako patent nr U.S. 5696079, który jest Krajowym Etapem zgłoszonego na podstawie 35 USC 371 zgłoszenia PCT/US94/05397, złożonego 19 maja 1994 r., którego treść jest włączona do niniejszego dokumentu przez przypis.
Zgłoszono 19 maja 1993 r.
Wydano Ostateczne Odrzucenie 20 stycznia 1998 r. odrzucony po odstąpieniu 14 sierpnia 1998 r. i 12 kwietnia 1999 r. ograniczone i zmodyfikowane roszczenia przyznane 8 maja 2000 r.
Ta rodzina patentów była podstawą kłamstwa Fauci’ego dla czasopisma British Medical Journal, w którym fałszywie stwierdził:
„Dr Anthony Fauci powiedział BMJ, że jako pracownik rządowy, był zobowiązany przez prawo do umieszczenia swojego nazwiska na patencie dotyczącym rozwoju interleukiny 2 [IL-2] i był również zobowiązany przez prawo do otrzymania części zapłaty, którą rząd otrzymał za wykorzystanie patentu. Powiedział, że uważa otrzymanie zapłaty za niestosowne (sic) i przekazał całą kwotę na cele charytatywne.” – BMJ. 2005 Jan 22; 330(7484): 162.
Nie był „zobowiązany przez prawo” do popełnienia oszustwa wobec urzędu patentowego, a następnie otrzymania za to zapłaty!
Patent US 6911527 Peptydy związane z HIV
Wynalazek ten polega na odkryciu nowych specyficznych epitopów i przeciwciał związanych z długotrwałym przeżyciem infekcji HIV-1. Te epitopy i przeciwciała mają zastosowanie w przygotowywaniu szczepionek do zapobiegania infekcji HIV-1 lub do kontrolowania progresji do AIDS.
Zgłoszony 6 maja 1999 r.
Odrzucony jako niepatentowalny 22 stycznia 2003 roku. Wydane z ostatecznym odrzuceniem 15 lipca 2004 r. po złożeniu wniosków o ponowne rozpatrzenie. Zmodyfikowane i ograniczone roszczenia dopuszczone 29 września 2004 roku.
Patent US 7368114 Białko fuzyjne, w tym CD4
Odkryto tu nowe rekombinowane polipeptydy, które zawierają polipeptyd CD4 podwiązany na jego końcu C z częścią immunoglobuliny zawierającą region zawiasowy i stałą domenę łańcucha ciężkiego immunoglobuliny ssaków.
Część lub IgG jest połączona na swoim końcu C z polipeptydem zawierającym końcówkę z końcem C łańcucha ciężkiego przeciwciała IgA lub końcówkę z końca C łańcucha ciężkiego przeciwciała IgM. Ujawnione są tu również metody stosowania tych białek fuzyjnych CD4.
Zgłoszono 24 października 2002 r.
Odrzucony jako niepatentowalny 18 sierpnia 2006 roku. Opłacone odwołanie w celu uchylenia ustaleń eksperta 15 lutego 2007 roku. Ponownie odrzucony 11 maja 2007 roku. W dniu 10 października 2007 r. wnioskodawcy jeszcze bardziej zawęzili konstrukcję tego, co wyraźnie nie było patentem, a USPTO przyznało mniej niż połowę roszczeń, o które ubiegano się w pierwotnym zgłoszeniu.
Patent US 9896509, 9193790 i 9441041 Zastosowanie antagonistów interakcji pomiędzy HIV GP120 i integryną alfa 4 beta 7
Podano metody leczenia zakażenia HIV. Metody mogą obejmować podawanie podmiotowi z zakażeniem HIV terapeutycznie skutecznej ilości środka, który zakłóca interakcję gp120 i alfa 4 integryny, takiego jak antagonista alfa 4 beta 1 lub integryny alfa 4 beta 7, lecząc w ten sposób zakażenie HIV. W kilku przykładach, antagonistą integryny alfa 4 jest przeciwciało monoklonalne, które wiąże się swoiście z podjednostką integryny alfa 4, beta 1 lub beta7 lub cyklicznym heksapeptydem o sekwencji aminokwasowej CWLDVC. Przedstawiono również metody zmniejszania replikacji lub infekcji HIV. Metody obejmują kontakt komórki ze skuteczną ilością środka, który zakłóca interakcję gp120 i integryny alfa 4, takiego jak antagonista integryny alfa 4 beta 1 lub alfa 4 beta 7. Ponadto, dostarczane są metody określania, czy środek jest użyteczny w leczeniu HIV.
Odrzucony 22 maja 2017 roku jako podwójnie opatentowany. W swojej odpowiedzi wnioskodawcy przyznają się do bezprawnego działania i dążą do uzyskania tylko tych elementów swojego zgłoszenia, które wykraczają poza okres obowiązywania wydanych patentów. W dniu 11 października 2017 r. wydano ograniczone roszczenia.
Próbka zagmatwanego przepływu funduszy, który wymyka się publicznemu ujawnieniu.
US Patent 8999351 został wydany Tekmira Pharmaceuticals Corporation w Burnaby, Kolumbia Brytyjska, w Kanadzie. W swoim patencie ujawniają oni, że ich badania były wspierane przez grant z Narodowego Instytutu Alergii i Chorób Zakaźnych (Grant HHSN266200600012C). Jak na ironię, ten grant o wartości 23 milionów dolarów został przyznany w 2006 roku firmie Alnylam Pharmaceuticals, Inc, a nie firmie Tekmira.
W 2012 roku firma Alnylam zgodziła się zapłacić firmie Tekmira 65 milionów dolarów w celu rozstrzygnięcia sporów prawnych, w tym roszczenia o odszkodowanie w wysokości 1 miliarda dolarów za „bezlitosne i okrutne” przywłaszczenie tajemnic handlowych firmy Tekmira. Od najwcześniejszego pierwszeństwa zgłoszenia patentowego z 10 listopada 2008 r., nie ma żadnego publicznego zapisu wskazującego na firmę Tekmira jako beneficjenta tego grantu NIAID. Niezależnie od tego, technologia nanocząstek lipidowych opracowana w ramach tego grantu jest technologią wykorzystywaną obecnie w interwencji [szprycy] firmy Moderna na COVID-19. W swoim zgłoszeniu 10-Q firma Alnylam informuje, że posiada licencję na technologię od firmy Arbutus – dawniej Tekmira – która oskarżyła firmę Acuitas o przywłaszczenie tajemnic handlowych i przekazanie licencji na nie firmie Moderna oraz koncernowi Pfizer współpracującego z firmą BioNTech.
Dodatkowe przypisy:
https://www.ott.nih.gov/nih-and-its-role-technology-transfer
https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/206288Orig1s000TAltr.pdf
https://www.gao.gov/assets/720/710287.pdf
https://grantome.com/search?q=%22National%20Institute%20of%20Allergy%20and%20Infectious%20Diseases%22
15 U.S.C. §1-3 – Spiskowanie w Celu Prowadzenia Przestępczej Działalności Gospodarczej
Każda umowa, kombinacja w formie powiernictwa lub w inny sposób, lub zmowa, w celu ograniczenia handlu pomiędzy kilkoma Stanami lub z obcymi narodami, jest uznana za nielegalną. Każda osoba, która zawrze jakąkolwiek umowę lub zaangażuje się w jakąkolwiek kombinację lub zmowę uznaną niniejszym za nielegalną, zostanie uznana za winną przestępstwa, a po skazaniu będzie podlegać karze grzywny nieprzekraczającej 100.000.000 dolarów, jeśli jest korporacją, lub, 1.000.000 dolarów jeśli jest inną osobą, lub karze pozbawienia wolności nieprzekraczającej 10 lat, lub obu tym karom, według uznania sądu.
Grant AI23946-08 z Narodowego Instytutu Zdrowia przyznany dr Ralphowi Baricowi z Uniwersytetu Północnej Karoliny w Chapel Hill (oficjalnie sklasyfikowany jako powiązany z NIAID dr Anthony’ego Fauci przynajmniej w 2003 roku) rozpoczął prace nad syntetycznym modyfikowaniem Coronaviridae (rodzina koronawirusów) w wyraźnym celu prowadzenia ogólnych badań, wzmacniania patogenności, wykrywania, manipulowania i potencjalnych interwencji terapeutycznych skierowanych na te same czynniki/patogeny. Już 21 maja 2000 roku dr R. Baric z Uniwersytetem Północnej Karoliny [UNC] dążyli do opatentowania krytycznych sekcji rodziny koronawirusów dla własnych korzyści komercyjnych [U.S. Provisional Application No. 60/206,537, filed May 21, 2000].
W jednej z kilku prac powstałych w wyniku prac sponsorowanych przez ten grant, dr Baric opublikował to, co według niego było pełnej długości cDNA wirusa SARS CoV, w którym wyraźnie stwierdzono, że wirus SAR CoV opierał się na kompozycji segmentów DNA.
„Używając panelu sąsiadujących cDNA, które obejmują cały genom, złożyliśmy cDNA o pełnej długości szczepu SARS-CoVUrbani i uratowaliśmy sklonowane molekularnie wirusy SARS (klon zakaźny SARS-CoV), które zawierały oczekiwane mutacje markera wstawione do klonów składowych.” – PNAS October 28, 2003 100 (22) 12995-13000; Reverse genetics with a full-length infectiousc DNA of severe acute respiratory syndrome coronavirus
Dnia 19 kwietnia 2002 roku – wiosną przed pierwszym wybuchem SARS w Azji – Christopher M. Curtis, Boyd Yount i Ralph Baric złożyli wniosek o patent nr US 7279372 na metodę produkcji rekombinowanego koronawirusa. W pierwszym publicznym zapisie roszczeń, starali się oni opatentować sposób wytwarzania „zakaźnego, defektywnego w replikacji koronawirusa”. Praca ta była wspierana przez grant NIH wspomniany powyżej i GM63228. W skrócie, amerykański Departament Zdrowia i Opieki Społecznej [HHS] był zaangażowany w finansowanie wzmacniania zakaźnej natury koronawirusa w latach 1999-2002, zanim SARS został kiedykolwiek wykryty u ludzi.
Na tym tle zauważyliśmy niezwykłe starania amerykańskiego Centrum Kontroli i Prewencji Chorób [CDC] na opatentowanie koronawirusa SARS wyizolowanego z ludzi, który według doniesień został przeniesiony na ludzi podczas epidemii SARS w Azji w latach 2002-2003. Rozdział 11, paragraf 101 Kodeksu Stanów Zjednoczonych [35 U.S.C. §101] zabrania patentowania przyrody. Ten przepis nie powstrzymał CDC w ich wysiłkach. Ich wniosek, uaktualniony w 2007 roku, ostatecznie został wydany jako patent US 7220852 i ograniczał każdego, kto nie był licencjonowany przez ich patent, od manipulowania SARS CoV, rozwijania testów lub zestawów do pomiaru koronawirusa SARS u ludzi lub pracy z ich opatentowanym wirusem do użytku terapeutycznego. Prace związane z tym wirusem, prowadzone przez wybranych współpracowników, obejmowały znaczne ilości inżynierii chimerycznej, badania typu uzyskiwania funkcji [gain-of-function], charakterystykę wirusa, wykrywanie, leczenie (zarówno szczepionki, jak i interwencje terapeutyczne) oraz badania nad bronią biologiczną.
Krótko mówiąc, dzięki patentowi dr Barica US 6593111 (roszczenia 1 i 5) oraz patentowi CDC US 7220852 (roszczenie 1), żadne badania w Stanach Zjednoczonych nie mogły być prowadzone bez ich zezwolenia lub naruszenia tego patentu.
Zauważyliśmy, że specjalista w dziedzinie badań typu gain-of-function, dr Ralph Baric, był zarówno odbiorcą milionów dolarów amerykańskich dotacji na badania od kilku instytucji federalnych, jak i zasiadał w Międzynarodowym Komitecie Taksonomii Wirusów (ICTV) Światowej Organizacji Zdrowia oraz w Grupie Badawczej Coronaviridae (CSG). W ramach tej funkcji był zarówno odpowiedzialny za określanie „nowości” kladów gatunków wirusów, jak i bezpośrednio korzystał z deklaracji o nowościach w postaci nowych zezwoleń na finansowanie badań oraz związanej z nimi współpracy patentowej i komercyjnej. Razem z CDC, NIAID, WHO, placówkami akademickimi i podmiotami komercyjnymi (w tym Johnson & Johnson; Sanofi i ich kilkoma firmami biotechnologicznymi posiadającymi patenty na koronawirusy; Moderna; Ridgeback; Gilead; Sherlock Biosciences; i, inne), potężna grupa interesów tworzyła to, co sugerowalibyśmy jako „wzajemnympowiązaniem dyrekcji” zgodnie z amerykańskimi prawem antymonopolowym.
1986-1990 NIAID Grant AI 23946 prowadzący do uzyskania patentu US 7279327 „Methods for Producing Recombinant Coronavirus”. Zgłoszony w 2002 r. i wydany w 2007 roku
Praca opublikowana po raz pierwszy na podstawie grantu NIAID to:
An Experimental Model for Dilated Cardiomyopathy after Rabbit Coronavirus Infection
https://europepmc.org/backend/ptpmcrender.fcgi?accid=PMC7109931&blobtype=pdf
1990 Pfizer zgłasza patent US 6372224 na szczepionkę przeciwko koronawirusowi opartą o białko S 14 listopada 2000 r. który został porzucony w kwietniu 2010 r., co czyni go domeną publiczną.
Lata 90. Prace koncentrowały się na związku koronawirusów z kardiomiopatią (patrz wyżej)
Wczesne odniesienie do „pojawienia się” koronawirusa jako patogenu układu oddechowego:
High Recombination And Mutation Rates In Mouse Hepatitis Virus Suggest That Coronaviruses May Be Potentiall Y Important Emerging Viruses
https://link.springer.com/content/pdf/10.1007%2F978-1-4615-1899-0_91.pdf
2000Dr Ralph Baric w ramach grantów AI23946 i GM63228 z Narodowego Instytutu Zdrowia [NIH] aktywnie pracuje nad rekombinowanym koronawirusem
2001 Narodowy Instytut Zdrowia, Alergie i Choroby Zakaźne. „Reverse Genetics with a Coronavirus Infectious cDNA Construct”. 4/1/2001-3/31/005 całkowity roczny koszty to 1 milion dolarów. RS Baric, PI
2002 Wybuch epidemii koronawirusa SARS w Azji
2003 25 kwietnia 2003 CDC złożyło patent, który ostatecznie uzyskała, jego numer to US 7220852 (patent na sekwencję RNA) oraz US 7776521 (patent na metodologię badań). Patenty te dają amerykańskiemu Departamentowi Zdrowia i Usług Społecznych [HHS] możliwość kontrolowania komercyjnego wykorzystania koronawirusa SARS.
Dr Anthony Fauci zostaje mianowany członkiem Naukowej Rady Doradczej Global Grand Challenges Fundacji Billa i Melindy Gatesów (pełnił tę funkcję do 2010 roku).
28 kwietnia 2003 r. Sequoia Pharmaceuticals składa wniosek o patent US 7151163(Środki przeciwwirusowe do leczenia, kontroli i zapobiegania zakażeniom koronawirusami). Otrzymało w tym samym roku dwa granty w wysokości 953 tysięcy dolarów od HHS.
21 lipca 2003 r. zespół Ralpha Barica (wykorzystujący granty AI23946 i GM63228) zgłasza patent US 7618802 (Kompozycje koronawirusów z genomem odpornym na rekombinację), który został wydany 17 listopada 2009 roku.
Instytut Onkologii Dana-Farber w listopadzie 2003 zgłasza patent US 7750123 na przeciwciało monoklonalne neutralizujące wirusa SARS CoV. Badania te są wspierane przez kilka grantów NIH, w tym przez granty z Narodowego Instytutu Zdrowia o oznaczeniach A128785, A148436 i A1053822.
2004 6 stycznia 2004 – SARS i bioterroryzm na konferencji Bioterrorism and Emerging Infectious Diseases: antimicrobials, therapeutics and immune modulators.
Na tej konferencji termin „Nowa Normalność” został wprowadzony przez firmę Merck.
Dr FAUCI I dr BARIC zaczynają zarabiać pieniądze!!! Narodowy Instytut Alergii i Chorób Zakaźnych (NIAID). SARS Reverse Genetics. AI059136-01. Koszty całkowite 1,7 miliona dolarów, RS Baric, PI. 10% wysiłku. 4/1/04- 3/31/09. Projekt ma na celu opracowanie pełnej długości zakaźnego cDNA wirusa SARS-CoV, opracowanie cząstek replikonowych SARS-CoV wyrażających heterologiczne geny oraz adaptację SARS-CoV na myszy, co pozwoli na stworzenie mysiego modelu patogennej infekcji SARS-CoV.
Narodowy Instytut Alergii i Chorób Zakaźnych. R01. Remodeling the SARS Coronavirus Genome Regulatory Network. RS Baric, PI 10% nakładu pracy. 7/1/04-6/30/09. 2,1 miliona dolarów.
22 listopada 2004 Uniwersytet w Hong Kongu patentuje białko kolcowe związane z SARS i ubiega się o patent US 7491489 (Syntetyczny peptyd celujący w krytyczne miejsca na białku kolca koronawirusa związanym z SARS odpowiedzialnym za infekcję wirusową i sposób jego zastosowania).
2005 Agencja ds. Zaawansowanych Projektów Badawczych w Obszarze Obronności (DARPA).Biohacking: Biological Warfare Enabling Technologies, czerwiec 2005. Waszyngton, Dystrykt Kolumbia. Wydarzenie sponsorowane przez DARPA/MITRE.
Przegląd osi czasu z https://www.youtube.com/watch?v=rO EeYBQiQU i https://www.davidmartin.world/wp-content/uploads/2020/04/20APRBotWslides.pdf
2008 Grant w dziedzinie obrony przed bronią biologicznąU54 AI057157 rozpoczyna się od 10.189.682 milionów dolarów dla UNC w Chapel Hill https://taggs.hhs.gov/Detail/AwardDetail?arg_awardNum=U54AI057157&arg_ProgOfficeCode=104
2009 Grant w dziedzinie obrony przed bronią biologicznąU54 AI057157 jest kontynuowany, przekazano kolejne 5.448.656 dolarów dla UNC w Chapel Hill (niekonkurencyjny grant od NIAID)
2010 Grant w dziedzinie obrony przed bronią biologiczną U54 AI057157 jest kontynuowany, przekazano 8.747.142 dolarów dla UNC w Chapel Hill (niekonkurencyjny grant od NIAID).
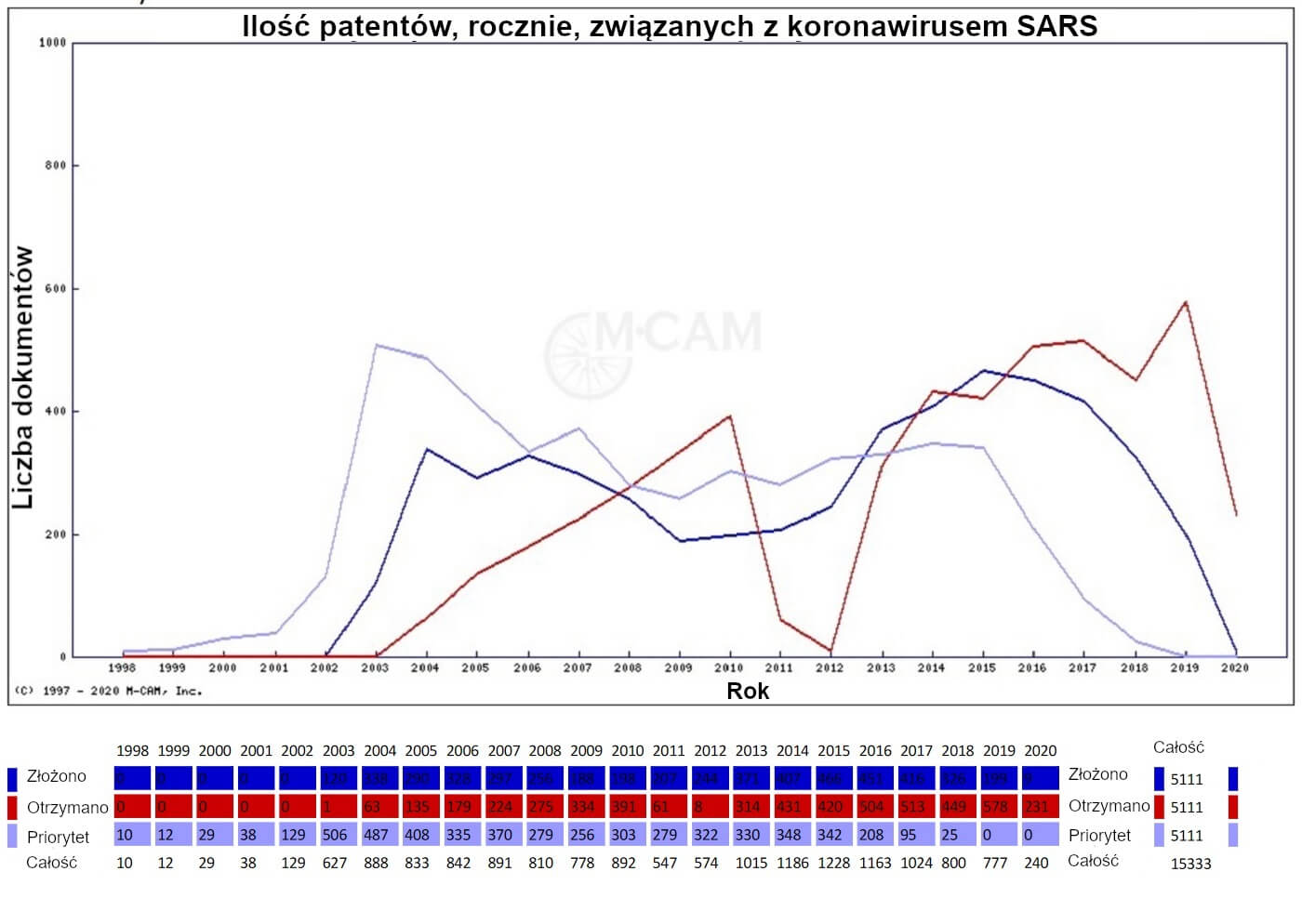
Liczba wydanych patentów na koronawirus SARS osiągnęła szczyt od czasu wybuchu epidemii w Azji (391 patentów).
6 sierpnia 2010 r., Moderna (przed jej założeniem) składa wniosek o patent US 9447164, który przyciągnął inwestorów (i „wynalazców”) z Flagship Ventures. Patent ten wyrósł z pracy dr Jasona P. Schruma z Harvard Medical School, wspieranego przez National Science Foundation Grant#0434507. Podczas gdy wniosek rości sobie prawo do pierwszeństwa z sierpnia 2010 roku, wniosek nie został sfinalizowany aż do października 2015 roku. W dniu 4 listopada 2015 roku USPTO wydał nieostateczne odrzucenie tego oryginalnego patentu, odrzucając wszystkie roszczenia.
https://www.nsf.gov/awardsearch/showAward?AWD_ID=0434507 z odniesieniem do finansowania w
https://molbio.mgh.harvard.edu/szostakweb/publications/Szostak_pdfs/Schrum_et_al_JACS_2009.pdf
2011 Crucell dołączył do Janssen Pharmaceutical Companies będącego częścią Johnson & Johnson w lutym, zabierając ze sobą całą swoją technologię dotyczącą SARS.
Grant w dziedzinie obrony przed bronią biologicznąU54 AI057157 jest kontynuowany, przekazano 7.344.820 dolarów dla UNC w Chapel Hill (niekonkurencyjny grant od NIAID).
2012 MERS wyizolowany w Egipcie
Grant w dziedzinie obrony przed bronią biologiczną U54 AI057157 jest kontynuowany, przekazano 7.627.657 USD dla UNC w Chapel Hill (niekonkurencyjny grant od NIAID).
2013 Grant w dziedzinie obrony przed bronią biologiczną U54 AI057157 jest kontynuowany, przekazano 7.226.237 milionów dolarów dla UNC w Chapel Hill (niekonkurencyjny grant od NIAID)
2014 23 kwietnia 2014, Moderna zgłasza patent na szczepionkę na bazie kwasu nukleinowego, patenty US9872900 i US10022435
2015 Moderna podpisuje umowę o rozwoju szczepionki z NIAID i realizuje ją z wiodącym na rynku twórcą i wynalazcą mRNA-1273 Guiseppe Ciaramella.
https://www.documentcloud.org/documents/6935295-NIH-Moderna-Confidential-Agreements.html
2016 Narodowy Instytut Zdrowia [NIH] poprzez Scripps Institute i Dartmouth College składają wniosek patentowy WO 2018081318A1 „Prefusion Coronavirus Spike Proteins and their Use [Białka kolcowe koronawirusa sprzed fuzji i ich zastosowanie]” ujawniający technologię mRNA, która pokrywa się (i jest używana w tandemie z) technologią Moderny.
Główny wynalazca Barney Scott Graham był dobrze znany firmie Moderna, jako że jest on osobą w Narodowym Instytucie Zdrowia [NIH], do której Moderna „wysłała e-maila” w celu uzyskania sekwencji dla SARS CoV-2, zgodnie z raportem Moderny tutaj („W styczniu 2020 roku, gdy odkryto, że infekcja w Wuhan była spowodowana nowym koronawirusem, Bancel szybko wysłał e-maila do dr Barneya Grahama, zastępcy dyrektora Centrum Badań nad Szczepionkami w Narodowym Instytucie Zdrowia, prosząc go o przesłanie sekwencji genetycznej wirusa.” – Źródło: Executives of vaccine developer Moderna cash in, cutcorners, 26 maj 2020)
Ponadto, współwynalazca Jason McLellan pracował z dr Barney’em Grahamem nad patentem na szczepionkę będącą wspólną własnością rządu chińskiego złożonym w Australii w 2013 roku. AU 2014231357A1 – Epitope of RSV fusion protein and antibody identifying same
2017 sierpień – Sanofi kupuje firmę Protein Science Corp posiadającą znaczne zasoby patentów na SARS
2018 czerwiec – firma Sanofi kupuje firmę Ablynx posiadającą znaczne zasoby patentów na SARS
2019 marzec, Sherlock Biosciences licencjonuje technologię Wyss, aby tworzyć przystępną cenowo diagnostykę molekularną – finansowane przez Open Philanthropy – tę samą organizację, która będzie sponsorem finansowym ćwiczeń „przy stole” o nazwie Wydarzeni 201, które ułożyło plan „pandemii” w październiku 2019 roku.
15 U.S.C. §8 – Manipulacja i Alokacja Rynku
Za sprzeczne z porządkiem publicznym, nielegalne i nieważne uznaje się wszelkie kombinacje, zmowy, powiernictwa, porozumienia lub umowy zawierane przez lub pomiędzy dwiema lub więcej osobami lub korporacjami, z których każda, jako agent lub zleceniodawca, zajmuje się importem jakiegokolwiek artykułu z jakiegokolwiek obcego kraju do Stanów Zjednoczonych, oraz gdy takie kombinacje, zmowy, powiernictwa, porozumienia lub umowy mają na celu ograniczenie legalnego handlu lub wolnej konkurencji w legalnym handlu, lub zwiększenie ceny rynkowej w jakiejkolwiek części Stanów Zjednoczonych jakiegokolwiek artykułu lub artykułów importowanych lub przeznaczonych do importu. Porozumienie lub umowa mają na celu ograniczenie handlu zgodnego z prawem lub wolnej konkurencji w handlu zgodnym z prawem, lub zwiększenie ceny rynkowej w jakiejkolwiek części Stanów Zjednoczonych artykułu lub artykułów importowanych lub przeznaczonych do importu do Stanów Zjednoczonych, lub jakiejkolwiek produkcji, do której taki importowany artykuł wchodzi lub ma wejść. Każda osoba, która zajmuje się importem jakichkolwiek towarów z jakiegokolwiek obcego kraju, z naruszeniem przepisów niniejszej sekcji, lub która łączy się lub spiskuje z innymi w celu naruszenia tych przepisów, jest winna wykroczenia i po skazaniu jej w jakimkolwiek sądzie Stanów Zjednoczonych taka osoba zostanie ukarana grzywną w wysokości nie mniejszej niż 100 dolarów i nie przekraczającej 5000 dolarów, a ponadto zostanie ukarana więzieniem, według uznania sądu, na okres nie krótszy niż trzy miesiące i nie przekraczający dwunastu miesięcy.
Poprzez niekonkurencyjne przyznawanie grantów dr Ralphowi Baricowi z Uniwersytetu Północnej Karoliny [UNC]w Chapel Hill, wybór lokalizacji laboratoriów bezpieczeństwa biologicznego poziomu 4 (BSL-4), ustalanie cen Remdesiviru i terapii mRNA firm Moderna i Pfizer, NIAID, CDC i Departament Zdrowia i Opieki Społecznej [HHS] były zaangażowane w przydzielanie funduszy federalnych spiskującym stronom bez niezależnej kontroli.
Od około 12 marca 2020 roku, w celu wzbogacenia własnych interesów ekonomicznych poprzez zapewnienie dodatkowych funduszy zarówno od podmiotów federalnych, jak i fundacji, CDC i dr Fauci z NIAID postanowili zawiesić badania i sklasyfikować COVID-19 wyłącznie na podstawie kapryśnej prezentacji objawów. Zmuszając opinię publiczną do polegania na The COVID Tracking Project – finansowanym przez Fundację Bloomberga, Zuckerberga i Gatesa i prezentowanym przez media (The Atlantic) – a nie agencję zdrowia publicznego – dr Fauci użył oszukańczej technologii testowania (RT-PCR), aby mylić „przypadki COVID” z pozytywnymi testami PCR u żywych, jednocześnie nalegając, aby zgony z powodu COVID były liczone wyłącznie na podstawie objawów. To utrwaliło popyt rynkowy na jego pożądany program szczepień, który był powtarzany przez niego i spiskujące z nim strony na całym świecie aż do chwili obecnej. Nic dziwnego, że było to konieczne z powodu widocznego spadku liczby przypadków, które stanowiły kryteria dr Fauci’ego i innych do pozbawiania obywateli ich praw wynikających z 1. Poprawki Konstytucji.
Myślenie pojęciowe, a myślenie stereotypowe – Andrzej Wronka, Kazimierz Ajdukiewicz
COVID – dlaczego terminologia ma znaczenie? – dr Malcolm Kendrick
Jak dokładne są testy na COVID? – dr Sebastian Rushworth
Testy PCR: Po 35 cyklach odcięcia nie wyhodujesz wirusa – dr Anthony Fauci
Nawet połowa testów na koronowirusa może być fałszywie pozytywna [Chiny]
Nieoczekiwane wykrycie przeciwciał na SARS-CoV-2 w okresie przed pandemicznym we Włoszech. [wrzesień 2019]
15 U.S.C. § 19 – Wzajemne Powiązania Między Dyrekcjami
(1) Żadna osoba nie może w tym samym czasie pełnić funkcji dyrektora lub urzędnika w dwóch korporacjach (innych niż banki, stowarzyszenia bankowe i spółki powiernicze), które są-
(A) zaangażowane w całości lub częściowo w handel; oraz
(B) ze względu na swoją działalność i miejsce prowadzenia działalności są konkurentami, tak że wyeliminowanie konkurencji w drodze porozumienia między nimi stanowiłoby naruszenie jakichkolwiek przepisów antymonopolowych; jeżeli każda z tych korporacji posiada kapitał, nadwyżkę i niepodzielne zyski o łącznej wartości przekraczającej 10.000.000 dolarów, skorygowane zgodnie z ustępem (5) niniejszej podsekcji.
Dr Anthony Fauci jest członkiem Rady Przywódczej [Leadership Council] Globalnego Planu Działań na rzecz Szczepionek [Gates Global Vaccine Action Plan] Billa i Malindy Gates.
Dr Fauci, kontrolując wydawanie federalnych funduszy na badania, był i nadal jest członkiem Rady Monitorowania Globalnej Gotowości [Global Preparedness Monitoring Board] Światowej Organizacji Zdrowia. Dołączył do niego w tej radzie skonfliktowany darczyńca z Fundacji Billa i Melindy Gates, dr Chris Elias, oraz dr George F. Gao z Chińskiej Rady Państwowej ichniego Centrum Kontroli i Prewencji Chorób. Ta Rada Monitorowania Globalnej Gotowości [GPMB] przewidywała, że wszystkie państwa członkowskie muszą wziąć udział w globalnej symulacji uwolnienia patogenu układu oddechowego.
Dr Ralph Baric jest jednym z głównych beneficjentów funduszy federalnych USA, prowadzi ośrodek BSL-4 i zasiada w grupie roboczej International Committee on Taxonomy of Virus Corona viridae, której zadaniem jest potwierdzenie obecności lub jego braku patogenu, za co otrzymuje bezpośrednie wynagrodzenie.
Jak wspomniano w części dotyczącej naruszeń 18 U.S.C. § 1001 powyżej, istnieją liczne nieujawnione relacje handlowe pomiędzy naukowcami otrzymującymi finansowanie, ich instytucjami finansującymi oraz interesami handlowymi, w których występują ujawnione i nieujawnione warunki handlowe. Pełna lista wszystkich potencjalnie zaangażowanych stron jest wymieniona w poniższej części zatytułowanej „Podmioty komercyjne”.
Wydaje się, że w okresie egzekwowania patentów i po orzeczeniu Sądu Najwyższego potwierdzającym, że patenty na materiał genetyczny są sprzeczne z prawem, CDC i Narodowy Instytut Alergii i Chorób Zakaźnych kierowany przez Anthony’ego Fauci (dalej odpowiednio „NIAID” i „dr Fauci”) weszły w wymianę handlową między państwami (w tym m.in. współpracując z Ecohealth Alliance Inc. ) oraz z zagranicą (w szczególności z Instytutem Wirusologii w Wuhan oraz Chińską Akademią Nauk) za pośrednictwem grantu R01AI110964 z programu grantów Narodowego Instytutu Zdrowiaz 2014 r. i następnych, w celu wykorzystania swoich praw patentowych.
Ponadto wydaje się, że w okresie egzekwowania patentów i po orzeczeniu Sądu Najwyższego potwierdzającym, że patenty na materiał genetyczny były nielegalne, CDC i Narodowy Instytut Alergii i Chorób Zakaźnych (dalej „NIAID”) weszły w wymianę handlową między stanami (w tym, ale nie tylko, współpracując z Uniwersytetem Północnej Karoliny w Chapel Hill) oraz z obcymi narodami (w szczególności z Instytutem Wirusologii w Wuhan i Chińską Akademią Nauk reprezentowaną przez dr Shi Zheng-Li) poprzez U19AI109761 (dr Ralph S. Baric), U19AI107810 (Ralph S. Baric) oraz grant Narodowej Fundacji Nauk Przyrodniczych w Chinach o oznaczeniu 81290341 (ShiZheng-Li) i wsp. 2015-2016.
Ponadto wydaje się, że w okresie egzekwowania patentów i po orzeczeniu Sądu Najwyższego potwierdzającym, że patenty na materiał genetyczny były nielegalne, CDC i NIAID weszły w wymianę handlową między Stanami (w tym, ale nie tylko, współpracując z Uniwersytetem Północnej Karoliny w Chapel Hill) i z obcymi narodami w celu prowadzenia chimerycznej konstrukcji nowatorskiego materiału koronawirusowego o specyficznych właściwościach wirulencji przed, w trakcie i po określeniu przez Narodowy Instytut Zdrowia [NIH] w październiku 17, 2014, że praca ta nie była wystarczająco zrozumiała w odniesieniu dostandardów bezpieczeństwa biologicznego tej instytucji.
Wytworzony w laboratorium koronawirus wywołał debatę – Jef Akst [2015]
W tym dochodzeniu zakłada się, że CDC i jej współpracownicy byli:
a) w pełni świadomi prac wykonywanych przy użyciu ich opatentowanej technologii;
b) zawarli wyraźne lub dorozumiane umowy obejmujące licencjonowanie, lub inne wynagrodzenie; i, c) świadomie zaangażowali jeden lub więcej zagranicznych interesów, aby kontynuować eksploatację ich zastrzeżonej technologii, gdy Sąd Najwyższy Stanów Zjednoczonych potwierdził, że takie patenty były nielegalne i gdy Narodowy Instytut Zdrowia [NIH] wydał moratorium na takie badania.
Podobno w styczniu 2018 r. ambasada USA w Chinach wysłała śledczych do Instytutu Wirusologii w Wuhan i stwierdziła, że
„Podczas interakcji z naukowcami w laboratorium Instytucie w Wuhan zauważyli oni, że nowe laboratorium ma poważny niedobór odpowiednio wyszkolonych techników i badaczy potrzebnych do bezpiecznego prowadzenia tegolaboratorium wysokiego poziomu bezpieczeństwa [szczelności biologicznej].” Gazeta Washington Post podała, że informacje te zostały zawarte w depeszy z 19 stycznia 2018 roku. Ponad rok później, w czerwcu 2019 r., CDC przeprowadziło inspekcję w Instytucie Badań Medycznych Chorób Zakaźnych Armii Stanów Zjednoczonych w Fort Detrick (zwanym dalej „USAMRIID”) i nakazało jego zamknięcie po stwierdzeniu, że ich inspekcja znalazła zagrożenia dla bezpieczeństwa biologicznego. Publikacja w czasopiśmie Nature z 2003 r. (423(6936): 103) donosi o współpracy między CDC a USAMRIID w zakresie badań nad koronawirusami, po której nastąpiła późniejsza znacząca współpraca.
„Naukowcy z NIH i armii amerykańskiej połączyli siły w systematycznym programie badań przesiewowych, aby znaleźć kandydatów na leki do zwalczania ciężkiego ostrego zespołu oddechowego (SARS). Współpraca, która już trwa, została ogłoszona na zeszłotygodniowej Międzynarodowej Konferencji na temat Badań Antywirusowych w Savannah w stanie Georgia.” – Nature, 8 maj 2003, US Army join shunt for SARS drug
CDC, ze względu na to, co wydaje się być tym samym rodzajem obaw zidentyfikowanych w Wuhan, zdecydowało się kontynuować współpracę z rządem chińskim, zamykając jednocześnie placówkę armii amerykańskiej.
CDC zgłosiło pierwszy przypadek choroby podobnej do SARS-CoV w Stanach Zjednoczonych w styczniu 2020 r., przy czym Służba Wywiadu Epidemiologicznego CDC zgłosiła 650 przypadków klinicznych i 210 testów. Biorąc pod uwagę, że podejrzany patogen po raz pierwszy pojawił się w oficjalnych raportach 31 grudnia 2019 r., można jedynie stwierdzić, że CDC:
a) posiadało mechanizm i środki do przeprowadzenia testów potwierdzających istnienie „nowego koronawirusa”; lub
b) nie posiadało takiego mechanizmu i fałszywie ogłosiło taki komunikat w styczniu.
Naiwnością jest sugerować, że WHO lub CDC mogły wyprodukować i rozprowadzić testy na „nowy” patogen, kiedy ich własne późniejsze osiągnięcia w zakresie rozwoju i wdrażania testów okazały się niewiarygodne…
Zagrożenie pandemią i wycieki z laboratoriów: Samospełniające się przepowiednie – dr Martin Furmanski
Długa historia przypadkowych ucieczek laboratoryjnych potencjalnie pandemicznych patogenów jest ignorowana w reportażach na temat COVID-19 – Sam Husseini
35 U.S.C. §200 – 206 – Ujawnienie Interesu Rządowego/Państwowego
35 U.S.C. §202 (c)(6)
Zobowiązanie wykonawcy, w przypadku złożenia wniosku patentowego w Stanach Zjednoczonych przez wykonawcę lub w jego imieniu, lub przez jakiegokolwiek cesjonariusza wykonawcy, do umieszczenia w specyfikacji takiego wniosku i każdego patentu wydanego na jego podstawie, oświadczenia określającego, że wynalazek został dokonany przy wsparciu rządu i że rząd posiada pewne prawa do wynalazku.
Ponad 5000 patentów i wniosków patentowych zawierało odniesienie do koronawirusa SARS sięgające dat pierwszeństwa z 1998 roku. Są one streszczone poniżej.
23 lipca 2020 roku Komisja Patentowa i Odwoławcza Biura Patentów i Znaków Towarowych Stanów Zjednoczonych odrzuciła starania firmy Moderna o unieważnienie patentu US 8058069. Patent ten, będący własnością Arbutus Biopharma Corp (należącej głównie do Roivant Science Ltd), obejmuje nanocząstki lipidowe (LNP) wymagane jako medium do dostarczenia mRNA w szczepionce. Niektóre z podstawowych technologii zostały oparte na pracach wykonanych pierwotnie na Uniwersytecie Kolumbii Brytyjskiej i zostały po raz pierwszy licencjonowane w 1998 roku.
Szczepionki mRNA na COVID-19 – dr James Odell
mRNA-1273 – eksperymentalna szczepionka opracowana przez Modernę na COVID-19 – wykorzystuje technologię nanocząstek lipidowych [LNP], na którą firma Moderna uważała że ma licencje od Acuitas Therapeutics Inc, firmy utworzonej przez byłego dyrektora wcześniejszej firmy Tekmira, należącej do Arbutusa. Ta licencja nie upoważniała firmy Moderna do wykorzystania tej technologii w szczepionce COVID-19.
Firmy M-CAM i Knowledge Ecology International niezależnie potwierdziły, że Moderna naruszyła prawo amerykańskie, nie ujawniając udziału rządu USA w finansowaniu swoich patentów i wniosków patentowych. Chociaż zaniedbanie to ma wpływ na wszystkie z ponad 130 przyznanych amerykańskich patentów firmy Moderna, jest ono szczególnie problematyczne w przypadku amerykańskiego patentu US 10702600 (Beta coronavirus mRNA vaccine), który jest patentem odnoszącym się do, „informacyjnego/matrycowego kwasu rybonukleinowego (mRNA) zawierającego otwartą ramkę odczytu kodującą białko kolca [S] lub podjednostkę białka S betakoronawirusa (BetaCoV), sformułowanego w nanocząstce lipidowej”.
Konkretne roszczenia odnoszące się do kluczowego elementu koronawirusa SARS zostały opatentowane 28 marca 2019 roku – 9 miesięcy przed wybuchem epidemii SARS CoV-2! Zarówno patent, jak i finansowanie DARPA dla tej technologii zostały ujawnione w publikacji naukowej (New England Journal of Medicine), ale fundusze rządowe nie zostały wspomniane w patencie.
W 2013 r. w ramach programu Autonomiczna Diagnostyka Umożliwiająca Profilaktykę i Terapię [Autonomous Diagnostics to Enable Prevention and Therapeutics – ADEPT] przyznano grant firmie Moderna Therapeutics na opracowanie nowego typu szczepionki opartej na informacyjnym RNA [mRNA]. Pierwotny grant DARPA nosił numer W911NF-13-1-0417. Firma wykorzystała tę technologię do opracowania swojej szczepionki na COVID-19, która w momencie opublikowania tego dossier przechodziła I fazę badań klinicznych we współpracy z NIH.29
Zgodnie z przepisami Federal Acquisition Regulation (FAR), kontrahenci rządu federalnego muszą w ramach kontraktu przedstawić informacje dotyczące kwestii naruszenia własności intelektualnej. Zgodnie z FAR §27.201-1(c) i (d) rząd wymaga zarówno zawiadomienia o naruszeniu lub potencjalnym naruszeniu, jak i zachowania odpowiedzialności ekonomicznej za naruszenie patentu. Konkretnie, w FAR §52.227.3 (a),
„Wykonawca zabezpieczy rząd i jego urzędników, agentów i pracowników przed odpowiedzialnością, w tym kosztami, za naruszenie jakiegokolwiek patentu Stanów Zjednoczonych…”. Oprócz patentów cytowanych przez USPTO podczas badań, które doprowadziły do patentu US 10702600, M-CAM zidentyfikowało czternaście innych wydanych patentów poprzedzających patent US 10702600, które zostały wykorzystane przez egzaminatorów patentowych do ograniczenia patentów wynikających z tych samych finansowanych badań, w tym patentów poszukiwanych przez CureVac.
Krótko mówiąc, podczas gdy Moderna cieszy się setkami milionów dolarów finansowania i wsparcia ze strony Anthony’ego Fauci i jego NIAID, od początku swego istnienia była zaangażowana w nielegalną działalność patentową i okazywała pogardę dla amerykańskiego prawa patentowego. Co gorsza, rząd Stanów Zjednoczonych udzielił firmie Moderna wsparcia finansowego w obliczu nieujawnionego ryzyka naruszenia prawa, potencjalnie przyczyniając się do tego samego naruszenia, za które otrzymali odszkodowanie.
21 C.F.R. § 50.24 et seq., Nielegalne Badania Kliniczne
Prowadzenie badań medycznych (nawet w nagłych przypadkach) jest niezgodne z prawem bez podjęcia szeregu kroków w celu:
- Zorganizowania badań przez uprawnioną i instytucjonalną komisją rewizyjną;
- Zapewnienia świadomej zgody wszystkim uczestnikom, w tym oświadczenia o ryzyku i korzyściach; oraz,
- Zaangażowania się w konsultacje ze społecznością, w której badanie ma być przeprowadzone.
Dr Anthony Fauci wymusił na zdrowej populacji Stanów Zjednoczonych bezprawne badanie kliniczne, w którym amerykański Departament Zdrowia i Usług Społecznych [HHS] ekstrapoluje dane epidemiologiczne. Nie uzyskano ani nie zabezpieczono świadomej zgody na żaden z „medycznych środków zaradczych” narzuconych populacji i nie powołano żadnej niezależnej komisji rewizyjnej – zgodnie z definicją zawartą w ustawie.
Do kwietnia 2020 roku, oficjalne zalecenie Journal of the American Medical Association było jednoznaczne.
„Maski na twarz nie powinny być noszone przez zdrowe osoby w celu ochrony przed nabyciem infekcji dróg oddechowych, ponieważ nie ma dowodów sugerujących, że maski na twarz noszone przez zdrowe osoby są skuteczne w zapobieganiu chorobie.” – JAMA. 2020;323(15):1517-1518; MedicalMasks
Część tego braku dowodów w rzeczywistości wykazała, że płócienne maski na twarz w rzeczywistości zwiększyły liczbę zachorowań związanych z grypą.
Maski z tkanin: niebezpieczne dla twojego zdrowia? – prof. Raina MacIntyre [2015]
Wbrew ustaleniom naukowym, stany, hrabstwa i przedsiębiorstwa naruszyły wymogi prawne dotyczące ogłaszania medycznych środków zaradczych podczas sytuacji zagrożenia zdrowia publicznego, wyrażając „przekonanie”, że maski na twarz ograniczają rozprzestrzenianie się wirusa SARS CoV-2. Jak dotąd, żadne z badań nie potwierdziło, że maska zapobiega przenoszeniu lub zakażeniu się SARS CoV-2.
Wydychany aerozol i maski na twarz
Wszystkie strony nakazujące stosowanie masek na twarz nie tylko świadomie ignorują ugruntowaną wiedzę z badań empirycznych, ale angażują się w coś, co jest równoznaczne z badaniem klinicznym całej populacji. Do takiego wniosku prowadzi fakt, że stosowanie masek na twarz i zapadalność na COVID-19 są omawiane w artykułach medycznych [naukowych] promowanych przez Amerykańskie Centrum Kontroli i Prewencji Chorób i inne instytucje.
Dystans społeczny do 2 metrów był promowany jako środek zapobiegający przenoszeniu się wirusów grypy z osoby na osobę. Chociaż w jednym z badań wysunięto hipotezę, że do zakażenia może dojść w odległości do około 2 metrów, w badaniu tym wyraźnie stwierdzono, że nie testowano przenoszenia się wirusa między osobami, a żywotność wirusa w odległości 2 metrów nie była nawet przedmiotem badania. Nie powstrzymało to jednak przeinaczenia wyników badania, które wykorzystano jako podstawę dla niezweryfikowanego medycznego środka zaradczego, jakim jest dystans społeczny. Do tej pory żadne badanie nie wykazało skuteczności dystansu społecznego w modyfikowaniu przenoszenia wirusa SARS CoV-2. Urzędnicy zdrowia publicznego powołują się na publikację J InfectDis. 2013 Apr;207(7):1037-46:
„Wyniki: Piętnaście badań, w tym 12 modelujących i trzy epidemiologiczne, spełniło kryteria kwalifikacji. Badania epidemiologiczne wykazały, że dystans społeczny był związany z redukcją zachorowań na choroby grypopodobne i serokonwersji na grypę A (H1N1) z 2009 roku. Jednak ogólne ryzyko błędu systematycznego w badaniach epidemiologicznych było poważne. W badaniach modelujących oszacowano, że same środki dystansu społecznego w miejscu pracy spowodowały medianę obniżenia o 23% skumulowanego wskaźnika zachorowań na grypę w populacji ogólnej. Opóźniły one również i zmniejszyły szczytową częstotliwość ataków grypy. Redukcja skumulowanego wskaźnika ataków była bardziej wyraźna, gdy dystans społeczny w miejscu pracy był połączony z innymi interwencjami niefarmakologicznymi lub farmaceutycznymi. Oceniono jednak, że skuteczność zmniejszała się wraz z wyższymi wartościami podstawowej liczby reprodukcyjnej, opóźnieniem uruchomienia dystansu społecznego w miejscu pracy lub mniejszą zgodnością.
Wnioski: Badania modelowe przemawiają za stosowaniem dystansu społecznego w miejscach pracy niezwiązanych z opieką zdrowotną, ale brakuje dobrze zaprojektowanych badań epidemiologicznych.” – BMC Public Health. 2018; 18: 518; Effectiveness of workplace social distancing measures in reducing influenza transmission: a systematic review
Wbrew ustaleniom naukowym, państwa, powiaty i przedsiębiorstwa naruszyły wymogi prawne dotyczące ogłaszania medycznych środków zaradczych podczas zagrożenia zdrowia publicznego, wyrażając „przekonanie”, że dystans społeczny zdrowej populacji ogranicza rozprzestrzenianie się SARS CoV-2. Jak dotąd, ani jedno badanie nie potwierdziło, że dystansowanie społeczne jakiejkolwiek populacji zapobiegło rozprzestrzenianiu się lub zakażeniu SARS CoV-2.
Zgodnie z ustawą o Federalnej Komisji Handlu [FTC Act], Rozdział 15, paragraf 41 Kodeksu Stanów Zjednoczonych [15 U.S.C. § 41] i następne, niezgodne z prawem jest reklamowanie, że produkt lub usługa może zapobiegać, leczyć lub uzdrawiać choroby ludzkie, chyba że posiada się kompetentne i wiarygodne dowody naukowe, w tym, w stosownych przypadkach, rzetelne kontrolowane badania kliniczne z udziałem ludzi, potwierdzające, że twierdzenia te są prawdziwe w chwili ich wygłaszania. W związku z tym, każda strona promująca stosowanie maseczek na twarz, narusza Ustawę o Federalnej Komisji Handlu.
Wszystkie te przepisy zostały złamane. Wszystkie właściwe władze w Stanach Zjednoczonych muszą zaprzestać stosowania masek na twarz do czasu skorygowania powyższych spraw.
Podmioty komercyjne
Dla wielu osób koronawirus SARS jest nowym tematem. Od 1999 roku możliwość manipulacji i wykorzystania koronawirusów do różnych celów przyciągnęło uwagę wielu osób, instytucji i organizacji komercyjnych, zarówno w sektorze publicznym, prywatnym jak i non-profit. Poniżej znajduje się lista ponad 5100 patentów i wniosków patentowych, zgłoszonych w wyraźnym celu kontrolowania jakiegoś aspektu koronawirusa SARS.
| PATENT | Tytuł | Właściciel | Priorytet | Data złożenia | Data otrzymania |
| US9995706 | Amperometric gas sensor | Steris Corporation | 25-
Jun- 12 |
30-
Sep -14 |
12-
Jun- 18 |
| US9995705 | Amperometric gas sensor | Steris Corporation | 25-
Jun- 12 |
30-
Sep -14 |
12-
Jun- 18 |
| US9994558 | Multicyclic compounds and methods of using same | Karyopharm Therapeutics Inc. | 20-
Sep- 13 |
19-
Sep -14 |
12-
Jun- 18 |
| US9994550 | Heterocyclic modulators of lipid synthesis for use against cancer and viral infections | 3-V Biosciences, Inc. | 7-
Jan- 14 |
7-
Jan -15 |
12-
Jun- 18 |
| US9993543 | Immunogenic compositions comprising silicified virus and methods of use | Portland State University | 31-
Jan- 13 |
31-
Jan -14 |
12-
Jun- 18 |
| US9982257 | Chiral control | WAVE LIFE SCIENCES LTD. | 13-
Jul-12 |
12-
Jul- 13 |
29-
May -18 |
| US9982241 | Recombinant HCMV and RHCMV vectors and uses thereof | Oregon Health & Science University | 14-
May- 10 |
1-
Oct -15 |
29-
May -18 |
| US9982025 | Monomeric griffithsin tandemers | The United States of America, as represented by the Secretary, Department of Health and Human Services | 5-
Jun- 13 |
5-
Jun -14 |
29-
May -18 |
| US9981036 | Compositions, comprising improved Il-12 genetic constructs and vaccines, immunotherapeutics and methods of using the same | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | 12-
Dec- 11 |
26-
Feb -16 |
29-
May -18 |
| US9975885 | Broad-spectrum non-covalent coronavirus protease inhibitors | PURDUE RESEARCH FOUNDATION | 28-
Apr- 16 |
28-
Apr -17 |
22-
May -18 |
| US9974850 | Immunogenic compositions and uses thereof | BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM | 25-
Mar- 15 |
25-
Mar -16 |
22-
May -18 |
| US9974848 | Tetanus toxoid and CCL3 improve DC vaccines | Duke University | 14-
Nov- 13 |
14-
Nov -14 |
22-
May -18 |
| US9974845 | Combination of vaccination and inhibition of the PD-1 pathway | CureVac AG | 22-
Feb- 13 |
21-
Feb -14 |
22-
May -18 |
| US9970061 | Bioagent detection oligonucleotides | IBIS BIOSCIENCES, INC. | 27-
Dec- 11 |
27-
Dec -12 |
15-
May -18 |
| US9969793 | Compositions and methods for the treatment of immunodeficiency | ADMA Biologics, Inc. | 28-
Oct- 14 |
13-
Nov -17 |
15-
May -18 |
| US9963718 | LCMV-GP-VSV-pseudotyped vectors and tumor-infiltrating virus- producing cells for the therapy of tumors | VIRATHERAPEUTICS GMBH | 8-
Oct- 08 |
7-
Apr -17 |
8-
May -18 |
| US9963611 | Composition for use in decreasing the transmission of human pathogens | Innonix Technologies, Incorporated | 29-
May- 09 |
21-
May -10 |
8-
May -18 |
| US9963427 | Dithiol mucolytic agents | PARION SCIENCES, INC. | 23-
Aug- 13 |
11-
Mar -16 |
8-
May -18 |
| US9962439 | Injectable vaccine composition | NITTO DENKO CORPORATION | 3-
Oct- 13 |
2-
Oct -14 |
8-
May -18 |
| US9957302 | Treating cancer with viral nucleic acid | Mayo Foundation for Medical Education and Research | 20-
Feb- 07 |
6-
Jul- 15 |
1-
May -18 |
| US9957300 | Virus-like particles, methods of preparation, and immunogenic compositions | Emory University | 17-
May- 02 |
4-
May -15 |
1-
May -18 |
| US9957238 | Arylalkyl-and aryloxyalkyl-substituted epithelial sodium channel blocking compounds | Parion Sciences, Inc. | 13-
Dec- 13 |
1-
Mar -17 |
1-
May -18 |
| US9951317 | Highly efficient influenza matrix (M1) proteins | NOVAVAX, INC. | 11-
Jul-03 |
6-
Oct -16 |
24-
Apr- 18 |
| US9951124 | Antibody producing non-human mammals | MERUS N.V. | 27-
Jun- 08 |
25-
Jan -13 |
24-
Apr- 18 |
| US9951122 | Antibodies against influenza virus and methods of use thereof | BURNHAM INSTITUTE FOR MEDICAL RESEARCH | 6-
Dec- 07 |
12-
Aug -13 |
24-
Apr- 18 |
| US9950062 | Compounds and compositions as TLR activity modulators | GLAXOSMITHKLINE BIOLOGICALS SA | 2-
Sep- 09 |
1-
Sep -10 |
24-
Apr- 18 |
| US9945856 | Coronavirus, nucleic acid, protein, and methods for the generation of vaccine, medicaments and diagnostics | AMSTERDAM INSTITUTE OF VIRAL GENOMICS B.V. | 18-
Aug- 03 |
13-
Aug -14 |
17-
Apr- 18 |
| US9945780 | Use of a fluorescent material to detect failure or deteriorated performance of a fluorometer | GEN-PROBE INCORPORATED | 14-
Jun- 12 |
7-
Jun -13 |
17-
Apr- 18 |
| US9944928 | Construction of pool of interfering nucleic acids covering entire RNA target sequence and related compositions | York Yuan Yuan Zhu | 23-
Jul-07 |
2-
Jul- 15 |
17-
Apr- 18 |
| US9944695 | Antibody producing non-human mammals | Menus N.V. | 27-
Jun- 08 |
30-
Apr -14 |
17-
Apr- 18 |
| US9944686 | Treatment of tumors with recombinant interferon alpha | SUPERLAB FAR EAST LIMITED | 28-
Feb- 01 |
5-
Sep -13 |
17-
Apr- 18 |
| US9944649 | Compounds and compositions as toll-like receptor 7 agonists | Novartis Ag | 1-
May- 14 |
29-
Apr -15 |
17-
Apr- 18 |
| US9943614 | Cationic steroid antimicrobial diagnostic, detection, screening and imaging methods | BRIGHAM YOUNG UNIVERSITY | 17-
Jun- 08 |
16-
Jun -09 |
17-
Apr- 18 |
| US9938300 | Isothiazolopyrimidinones, pyrazolopyrimidinones, and pyrrolopyrimidinones as ubiquitin-specific protease 7 inhibitors | Forma Therapeutics, Inc. | 5-
Feb- 15 |
4-
Feb -16 |
10-
Apr- 18 |
| US9938275 | Substituted imidazoquinolines, imidazopyridines, and i midazonaphthyridines | 3M Innovative Properties Company | 18-
Jun- 04 |
23-
Jan -17 |
10-
Apr- 18 |
| US9938258 | Substituted 2,3-dihydrobenzofuranyl compounds and uses thereof | Karyopharm Therapeutics Inc. | 29-
Nov- 12 |
27-
Nov -13 |
10-
Apr- 18 |
| US9932351 | Thienopyrimidinones as ubiquitin-specific protease 7 inhibitors | Forma Therapeutics, Inc. | 5-
Feb- 15 |
4-
Feb -16 |
3-
Apr- 18 |
| US9932323 | Therapeutic hydroxypyridinones, hydroxypyrimidinones and hydroxypyridazinones | Rutgers, The State University of New Jersey | 11-
Sep- 12 |
13-
Jan -17 |
3-
Apr- 18 |
| US9931316 | Antiviral activity from medicinal mushrooms and their active constituents | Not Available | 31-
Mar- 15 |
14-
Sep -15 |
3-
Apr- 18 |
| US9926340 | NAD analogs and methods of using said NAD analogs in determining ribosylation of proteins with PARP mutants | Biolog Life Science Institute Forshungslabor und Biochemica-Vertrieb GmbH | 8-
Apr- 15 |
1-
Apr -16 |
27-
Mar -18 |
| US9925215 | Anionically modified polyallylamine derivative, use of anionically modified polyallylamine derivative as medicine, particularly for propylaxis and treatment of infections of respiratory tract caused by human metapneumovirus (hMPV), human rhinoviruses (HRV), and infection by influenza virus type A (IAV) and pharmaceutical composition comprising the anionically modified polyallylamine derivative | UNIWERSYTET JAGIELLONSKI | 29-
Jul-14 |
25-
Oct -17 |
27-
Mar -18 |
| US9920314 | Compositions for and methods of identifying antigens | President and Fellows of Harvard College | 21-
Feb- 06 |
6-
May -15 |
20-
Mar -18 |
| US9920128 | Synthetic antiserum for rapid-turnaround therapies | The Johns Hopkins University | 28-
Jan- 15 |
20-
Jan -16 |
20-
Mar -18 |
| US9919034 | Methods of treating and prophylactically protecting mammalian patients infected by viruses classified in Baltimore group V | TAMIR BIOTECHNOLOGY, INC. | 28-
Mar- 14 |
10-
Jun -15 |
20-
Mar -18 |
| US9915613 | Systems and methods for distinguishing optical signals of different modulation frequencies in an optical signal detector | GEN-PROBE INCORPORATED | 24-
Feb- 11 |
21-
Mar -14 |
13-
Mar -18 |
| US9914976 | Methods and compositions for prostate cancer metastasis | FLORIDA AGRICULTURAL AND MECHANICAL UNIVERSITY (FA | 25-
Mar- 11 |
27-
May -16 |
13-
Mar -18 |
| US9913801 | Treatment of evolving bacterial resistance diseases including Klebsiella pneumoniae with liposomally formulated glutathione | YOUR ENERGY SYSTEMS, LLC | 15-
Feb- 13 |
15-
Mar -13 |
13-
Mar -18 |
| US9909176 | Efficient deep sequencing and rapid genomic speciation of RNA viruses (vRNAseq) | The Johns Hopkins University | 8-
Sep- 14 |
1-
Sep -15 |
6-
Mar -18 |
| US9908946 | Generation of binding molecules | Merus N.V. | 26-
Sep- 11 |
16-
Sep -15 |
6-
Mar -18 |
| US9908675 | Powdered pouch and method of making same | MONOSOL, LLC | 16-
Apr- 12 |
19-
Jul- 16 |
6-
Mar -18 |
| US9907796 | Methods of treating tumoral diseases, or bacterial or viral infections | INHIBIKASE THERAPEUTICS, INC. | 4-
Oct- 12 |
15-
Sep -16 |
6-
Mar -18 |
| US9895692 | Sample-to-answer microfluidic cartridge | Micronics, Inc. | 29-
Jan- 10 |
5-
Aug -15 |
20-
Feb -18 |
| US9895411 | Analogs of C5a and methods of using same | BOARD OF REGENTS OF THE UNIVERSITY OF NEBRASKA | 29-
Jun- 10 |
29-
Jun -11 |
20-
Feb -18 |
| US9895341 | Inflammation and immunity treatments | Ocean Spray Cranberries, Inc. | 1-
Apr- 11 |
30-
Mar -12 |
20-
Feb -18 |
| US9894888 | Transgenic immunodeficient mouse expressing human SIRP-alpha | INSTITUT PASTEUR | 26-
Mar- 12 |
26-
Mar -13 |
20-
Feb -18 |
| US9890419 | Nanoreporters and methods of manufacturing and use thereof | NanoString Technologies, Inc. | 23-
Dec- 05 |
20-
May -16 |
13-
Feb -18 |
| US9890408 | Multiple displacement amplification | IBIS BIOSCIENCES, INC. | 15-
Oct- 09 |
15-
Oct -10 |
13-
Feb -18 |
| US9890362 | Compositions, methods and uses for inducing viral growth | Takeda Vaccines, Inc. | 5-
Dec- 08 |
19-
Sep -14 |
13-
Feb -18 |
US9890361 Methods for increasing the infectivity of viruses utilizing alkyne- modified fatty acids LIFE TECHNOLOGIES CORPORATION
26-Jan-12
25-Jan-13
13-Feb-18
US9890206 H1N1 flu virus neutralizing antibodies Medigen Biotechnology Corporation
20-Aug-15
20-Aug-15
13-Feb-18
US9890169 Triazolinone compounds as HNE inhibitors CHIESI FARMACEUTICI S.P.A.
14-Dec-15
12-Dec-16
13-Feb-18
US9890124 Benzazepine sulfonamide compounds Hoffmann-La Roche Inc.
15-Dec-15
14-Jun-17
13-Feb-18
US9889194 Immunogenic composition for MERS coronavirus infection New York Blood Center, Inc.
1-Mar-13
28-Feb-14
13-Feb-18
US9885092 Materials and methods for detection of HPV nucleic acids QIAGEN GAITHERSBURG INC.
24-Feb-11
23-Feb-12
6-Feb-18
US9885082 Embodiments of a probe and method for targeting nucleic acids University of Idaho
19-Jul-11
19-Jul-12
6-Feb-18
US9885037 Chiral control WAVE LIFE SCIENCES LTD.
13-Jul-12
12-Jul-13
6-Feb-18
US9884895 Methods and compositions for chimeric coronavirus spike proteins The University of North Carolina at Chapel Hill
20-Mar-14
20-Mar-15
6-Feb-18
US9884876 Anti-viral compounds, pharmaceutical compositions, and methods of use thereof Kineta, Inc.
9-May-14
8-May-15
6-Feb-18
US9884129 Release of agents from cells The Brigham and Women’s Hospital, Inc.
15-Oct-09
5-Jan-15
6-Feb-18
US9884032 Esters of short chains fatty acids for use in the treatment of immunogenic disorders PROPONENT BIOTECH GMBH
3-Oct-12
3-Mar-16
6-Feb-18
US9884026 Modular particles for immunotherapy YALE UNIVERSITY
1-Nov-13
31-Oct-14
6-Feb-18
US9880151 Method of determining, identifying or isolating cell-penetrating peptides Phylogica Limited
23-May-11
23-May-12
30-Jan-18
US9879026 Substituted spirocycles Boehringer Ingelheim International GmbH
12-Sep-14
29-Nov-16
30-Jan-18
US9879003 Host targeted inhibitors of dengue virus and other viruses Dana-Farber Cancer Institute, Inc.
11-Apr-12
15-Mar-13
30-Jan-18
US9878988 Dendrimer like amino amides possessing sodium channel blocker activity for the treatment of dry eye and other mucosal diseases PARION SCIENCES, INC.
29-May-12
5-Jan-16
30-Jan-18
US9873678 Chemical compounds AstraZeneca AB
18-Mar-14
17-Mar-15
23-Jan-18
US9873674 C-Rel inhibitors and uses thereof CORNELL UNIVERSITY
21-Sep-12
19-Sep-13
23-Jan-18
US9872900 Nucleic acid vaccines ModernaTX, Inc.
23-Apr-14
5-Apr-16
23-Jan-18
US9872898 Compositions and methods for treating and preventing porcine reproductive and respiratory syndrome Ohio State Innovation Founation
24-Apr-12
3-Oct-16
23-Jan-18
US9872895 TLR5 ligands, therapeutic methods, and compositions related thereto Emory University
24-Sep-10
20-Sep-11
23-Jan-18
| US9868952 | Compositions and methods for âCœresistance-proofâC SiRNA therapeutics for influenza | Sirnaomics, Inc. | 8-Jul-
12 |
7-
Jul- 13 |
16-
Jan- 18 |
| US9868740 | Pyrimidinone compounds which are HNE inhibitors | CHIESI FARMACEUTICI S.p.A. | 12-
Jun- 14 |
12-
Jun -14 |
16-
Jan- 18 |
| US9868736 | Deubiquitinase inhibitors and methods for use of the same | THE REGENTS OF THE UNIVERSITY OF MICHIGAN | 10-
Oct- 13 |
10-
Oct -14 |
16-
Jan- 18 |
| US9867882 | Carbohydrate conjugates as delivery agents for oligonucleotides | Alnylam Pharmaceuticals, Inc. | 4-
Dec- 07 |
25-
Aug -15 |
16-
Jan- 18 |
| US9867877 | Methods for preparing squalene | NOVARTIS AG | 12-
May- 10 |
22-
Nov -16 |
16-
Jan- 18 |
| US9862706 | Compounds | CHIESI FARMACEUTICI S.p.A. | 31-
May- 16 |
26-
May -17 |
9-
Jan- 18 |
| US9861614 | Nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 9-
May- 12 |
23-
Jun -15 |
9-
Jan- 18 |
| US9856254 | Alkoxy substituted imidazoquinolines | 3M Innovative Properties Company | 3-
Oct- 03 |
13-
Jun -16 |
2-
Jan- 18 |
| US9856241 | Substituted benzofuranyl and benzoxazolyl compounds and uses thereof | Karyopharm Therapeutics Inc. | 3-Jul-
13 |
3-
Jul- 14 |
2-
Jan- 18 |
| US9856228 | Peptidyl nitril compounds as dipeptidyl peptidase I inhibitors | PROZYMEX A/S | 9-
Sep- 13 |
8-
Sep -14 |
2-
Jan- 18 |
| US9856224 | Stable sodium channel blockers | PARION SCIENCES, INC. | 30-
Jun- 14 |
30-
Jan -17 |
2-
Jan- 18 |
| US9855287 | Anti-viral azide containing compounds | LIFE TECHNOLOGIES CORPORATION | 28-
Jul-10 |
20-
Aug -15 |
2-
Jan- 18 |
| US9855284 | Pharmaceutical compositions and methods | Pop Test Oncology LLC | 3-
Aug- 15 |
6-
Dec -16 |
2-
Jan- 18 |
| US9849143 | Broad spectrum antiviral and methods of use | The Burlington HC Research Group, Inc. | 17-
Apr- 06 |
16-
Feb -17 |
26-
Dec -17 |
| US9845342 | Fusion proteins, recombinant bacteria, and methods for using recombinant bacteria | Spogen Biotech Inc. | 17-
Sep- 14 |
17-
Sep -15 |
19-
Dec -17 |
| US9840731 | Preservation of biological materials in non-aqueous fluid media | Gentegra, LLC | 14-
Mar- 13 |
14-
Mar -14 |
12-
Dec -17 |
| US9840719 | Variant AAV and compositions, methods and uses for gene transfer to cells, organs and tissues | The Children’s Hospital of Philadelphia | 22-
Jul-13 |
22-
Jul- 14 |
12-
Dec -17 |
| US9840491 | Quinazolinones and azaquinazolinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
4-
Feb -16 |
12-
Dec -17 |
| US9839687 | Acetylenedicarboxyl linkers and their uses in specific conjugation of a cell-binding molecule | SUZHOU M-CONJ BIOTECH CO., LTD. | 15-
Jul-15 |
15-
Jul- 15 |
12-
Dec -17 |
| US9834812 | Probe kit for detecting a single strand target nucleotide sequence | Fondazione Istituto Italiano Di Tecnologia | 27-
Dec- 12 |
27-
Dec -13 |
5-
Dec -17 |
| US9834791 | CRISPR-related methods and compositions with governing gRNAS | Editas Medicine, Inc. | 7-
Nov- 13 |
7-
Nov -14 |
5-
Dec -17 |
| US9834757 | Hand, foot, and mouth vaccines and methods of manufacture and use thereof | Takeda Vaccines, Inc. | 7-
Nov- 14 |
6-
Nov -15 |
5-
Dec -17 |
| US9834595 | Amino acid sequences directed against envelope proteins of a virus and polypeptides comprising the same for the treatment of viral diseases | Ablynx N.V. | 5-
Jun- 08 |
29-
Oct -15 |
5-
Dec -17 |
| US9833504 | Virus-like particles and process for preparing same | Folia Biotech Inc. | 13-
May- 11 |
1-
May -12 |
5-
Dec -17 |
| US9833492 | Combinations of a caspase inhibitor and an antiviral agent | Centre National de la Recherche Scientifique | 2-
Nov- 07 |
15-
May -15 |
5-
Dec -17 |
| US9832998 | Antiviral compositions | Long Island University | 30-
May- 07 |
19-
Mar -15 |
5-
Dec -17 |
| US9828382 | Pyrimidinone compounds as human neutrophil elastase inhibitors | Chiesi Farmaceutici S.p.A. | 18-
Dec- 12 |
10-
May -16 |
28-
Nov -17 |
| US9828379 | Pyrrolo-pyrrole carbamate and related organic compounds, pharmaceutical compositions, and medical uses thereof | ABIDE THERAPEUTICS, INC. | 3-Jul-
13 |
1-
Jul- 14 |
28-
Nov -17 |
| US9828370 | Compositions and methods for inhibiting kinases | INHIBIKASE THERAPEUTICS, INC. | 23-
Apr- 15 |
22-
Apr -16 |
28-
Nov -17 |
| US9828346 | N-myristoyl transferase inhibitors | University of Dundee | 2-
Sep- 08 |
31-
Aug -15 |
28-
Nov -17 |
| US9828342 | Isatin derivatives, pharmaceutical compositions thereof, and methods of use thereof | CITY OF HOPE | 24-
Feb- 12 |
25-
Feb -13 |
28-
Nov -17 |
| US9827190 | Intradermal delivery of immunological compositions comprising tolllike receptor 7 agonists | GLAXOSMITHKLINE BIOLOGICALS SA | 1-
Feb- 13 |
30-
Jan -14 |
28-
Nov -17 |
| US9822339 | Means and methods for influencing the stability of antibody producing cells | ACADEMISCH MEDISCH CENTRUM BIJ DE UNIVERSITEIT VAN AMSTERDAM | 9-
Dec- 05 |
26-
Aug -15 |
21-
Nov -17 |
| US9822173 | Heterodimeric immunoglobulins | AMGEN INC. | 21-
Nov- 12 |
21-
Nov -13 |
21-
Nov -17 |
| US9822165 | Hydrocarbon stapled stabilized alpha-helices of the HIV-1 GP41 membrane proximal external region | DANA-FARBER CANCER INSTITUTE, INC. | 18-
Jun- 09 |
18-
Jun -10 |
21-
Nov -17 |
| US9822155 | Method of preventively treating a subject at the risk of developing infections of a respiratory virus | Xiangxue Group (Hong Kong) Company Limited | 9-
May- 13 |
23-
Aug -16 |
21-
Nov -17 |
| US9822127 | GAK modulators as antivirals | The Board of Trustees of the Leland Stanford Junior University | 23-
Jul-14 |
23-
Jul- 15 |
21-
Nov -17 |
| US9822065 | Benzazepine dicarboxamide compounds | Hoffmann-La Roche Inc. | 6-
Mar- 15 |
14-
Feb -17 |
21-
Nov -17 |
| US9821052 | Reverse genetics systems | Seqirus UK Limited | 31-
Jul-09 |
30-
Jul- 10 |
21-
Nov -17 |
| US9821051 | Reducing hospitalization in elderly influenza vaccine recipients | Seqirus UK Limited | 28-
Oct- 10 |
21-
Oct -11 |
21-
Nov -17 |
| US9816078 | Compositions for increasing polypeptide stability and activity, and related methods | SOLIS BIODYNE OAre | 19-
Nov- 09 |
11-
Mar -16 |
14-
Nov -17 |
| US9815886 | Compositions and methods for the treatment of immunodeficiency | ADMA BIOLOGICS, INC. | 28-
Oct- 14 |
8-
Jan -15 |
14-
Nov -17 |
| US9815805 | Certain (2S)-N-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2- carboxamides as dipeptidyl peptidase 1 inhibitors | ASTRAZENECA AB | 24-
Jan- 14 |
8-
Nov -16 |
14-
Nov -17 |
| US9814777 | Targeting lipids | Arbutus Biopharma Corporation | 4-
Dec- 07 |
22-
Oct -13 |
14-
Nov -17 |
| US9810683 | Use of live cell inteferometry with reflective floor of observation chamber to determine changes in mass of mammalian cells | The Regents of the University of California | 6-
May- 09 |
25-
Nov -13 |
7-
Nov -17 |
| US9809845 | Methods and reagents for amplifying nucleic acids | The United States of America, as represented by the Secretary, Department of Health and Human Services | 6-
Aug- 12 |
6-
Aug -12 |
7-
Nov -17 |
| US9809796 | Animal protein-free media for cultivation of cells | Baxalta GmbH | 29-
Oct- 04 |
18-
May -17 |
7-
Nov -17 |
| US9809632 | Universal protein tag for double stranded nucleic acid delivery | University of Washington Through its Center for Commercialization | 23-
Oct- 13 |
22-
Oct -14 |
7-
Nov -17 |
| US9809591 | Heterocyclic modulators of lipid synthesis | 3-V Biosciences, Inc. | 8-
Mar- 11 |
5-
Oct -15 |
7-
Nov -17 |
| US9808490 | Induced hepatocytes and uses thereof | ACCELERATED BIOSCIENCES CORP. | 26-
Nov- 14 |
25-
Nov -15 |
7-
Nov -17 |
| US9803236 | Microarray-based assay integrated with particles for analyzing molecular interactions | CapitalBio Corporation | 6-
Aug- 10 |
6-
Aug -10 |
31-
Oct- 17 |
| US9803197 | Particle-nucleic acid conjugates and therapeutic uses related thereto | Emory University | 25-
Jun- 12 |
27-
Feb -13 |
31-
Oct- 17 |
| US9802937 | Substituted pyrazolo{4,3-D}pyrimidines as kinase inhibitors | ORIGENIS GMBH | 21-
Apr- 11 |
23-
Apr -12 |
31-
Oct- 17 |
| US9802919 | Compounds | CHIESI FARMACEUTICI S.p.A. | 31-
May- 16 |
26-
May -17 |
31-
Oct- 17 |
| US9801948 | Antimicrobial compositions and methods of use thereof | Yale University | 21-
Sep- 11 |
21-
Sep -12 |
31-
Oct- 17 |
| US9801947 | Methods and compositions for enhancing immune response | 3M INNOVATIVE PROPERTIES COMPANY | 10-
Apr- 03 |
6-
Oct -14 |
31-
Oct- 17 |
| US9801935 | Soluble needle arrays for delivery of influenza vaccines | SEQIRUS UK LIMITED | 20-
Aug- 10 |
11-
Oct -16 |
31-
Oct- 17 |
| US9801897 | Delivery of RNA to trigger multiple immune pathways | GLAXOSMITHKLINE BIOLOGICALS SA | 6-Jul-
10 |
6-
Jul- 11 |
31-
Oct- 17 |
| US9797000 | Non-target amplification method for detection of RNA splice-forms in a sample | QIAGEN GAITHERSBURG INC. | 1-
May- 09 |
30-
Apr -10 |
24-
Oct- 17 |
| US9796979 | Oligonucleotide modulators of the toll-like receptor pathway | Quark Pharmaceuticals Inc. | 3-
Mar- 11 |
28-
Jul- 16 |
24-
Oct- 17 |
| US9796735 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 20-
Jun- 07 |
7-
Nov -14 |
24-
Oct- 17 |
| US9795669 | Lipidated immune response modifier compound compositions, formulations, and methods | 3M INNOVATIVE PROPERTIES COMPANY | 17-
Aug- 10 |
15-
Dec -15 |
24-
Oct- 17 |
| US9795668 | Delivery of self-replicating RNA using biodegradable polymer particles | GlaxoSmithKline Biologicals S.A. | 6-Jul-
10 |
23-
Nov -15 |
24-
Oct- 17 |
| US9795666 | High-yield transgenic mammalian expression system for generating virus-like particles | Academia Sinica | 5-
Sep- 06 |
11-
Feb -15 |
24-
Oct- 17 |
| US9791437 | Multianalyte assay | Nexus Dx, Inc. | 30-
Apr- 07 |
15-
Jun -15 |
17-
Oct- 17 |
| US9789180 | D-amino acid derivative-modified peptidoglycan and methods of use thereof | The Regents of the University of California | 30-
Nov- 12 |
31-
Mar -16 |
17-
Oct- 17 |
| US9786050 | Stain-free histopathology by chemical imaging | The Board of Trustees of the University of Illinois | 15-
Mar- 13 |
14-
Mar -14 |
10-
Oct- 17 |
| US9783595 | Neutralizing GP41 antibodies and their use | The United States of America, as represented by the Secretary, Department of Health and Human Services | 7-
Nov- 11 |
2-
Aug -16 |
10-
Oct- 17 |
| US9782470 | Method of obtaining thermostable dried vaccine formulations | Merck Sharp & Dohme Corp. | 16-
Oct- 13 |
13-
Oct -14 |
10-
Oct- 17 |
| US9782434 | Methods of treating or preventing inflammation and hypersensitivity with oxidative reductive potential water solution | Sonoma Pharmaceuticals, Inc. | 20-
Jan- 06 |
7-
Jul- 15 |
10-
Oct- 17 |
| US9770504 | Generating peptoid vaccines | The Board of Regents of the University of Texas System | 3-
May- 13 |
2-
May -14 |
26-
Sep -17 |
| US9770463 | Delivery of RNA to different cell types | GLAXOSMITHKLINE BIOLOGICALS SA | 6-Jul-
10 |
7-
Jun -11 |
26-
Sep -17 |
| US9765395 | System and method for DNA sequencing and blood chemistry analysis | Nanomedical Diagnostics, Inc. | 28-
Apr- 14 |
10-
Apr -15 |
19-
Sep -17 |
| US9765133 | Antibody producing non-human mammals | Merus N.V. | 27-
Jun- 08 |
29-
Apr -14 |
19-
Sep -17 |
| US9765071 | Substituted imidazo ring systems and methods | 3M INNOVATIVE PROPERTIES COMPANY | 25-
Nov- 03 |
14-
Mar -16 |
19-
Sep -17 |
| US9764027 | Outer membrane vesicles | GLAXOSMITHKLINE BIOLOGICALS SA | 18-
Sep- 12 |
18-
Sep -13 |
19-
Sep -17 |
| US9759723 | B-cell antigen presenting cell assay | University of Pittsburgha€”Of the Commonwealth System of Higher Education | 8-
Apr- 10 |
21-
Mar -16 |
12-
Sep -17 |
| US9758840 | Parasite detection via endosymbiont detection | IBIS BIOSCIENCES, INC. | 14-
Mar- 10 |
11-
Mar -11 |
12-
Sep -17 |
| US9758820 | Organism identification panel | BioFire Diagnostics, LLC | 2-
Apr- 07 |
1-
Apr -08 |
12-
Sep -17 |
| US9758775 | TAL effector-mediated DNA modification | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
14-
Apr -14 |
12-
Sep -17 |
| US9758568 | Oligopeptide-free cell culture media | Baxalta GmbH | 4-
Jan- 06 |
16-
Nov -15 |
12-
Sep -17 |
| US9758553 | Yeast strain for the production of proteins with terminal alpha-1,3- linked galactose | MERCK SHARP & DOHME CORP. | 30-
May- 08 |
2-
Jul- 14 |
12-
Sep -17 |
| US9757478 | Mutant protease biosensors with enhanced detection characteristics | Promega Corporation | 11-
May- 10 |
7-
Jan -16 |
12-
Sep -17 |
| US9757470 | Peptides for assisting delivery across the blood brain barrier | Children’s Medical Center Corporation | 22-
May- 06 |
30-
Apr -14 |
12-
Sep -17 |
| US9757446 | Influenza virus vectors and uses therefor | FLUGEN, INC. | 17-
Mar- 14 |
13-
Mar -15 |
12-
Sep -17 |
| US9757407 | T reatment of viral infections by modulation of host cell metabolic pathways | The Trustees of Princeton University | 1-
Jun- 07 |
21-
Dec -15 |
12-
Sep -17 |
| US9751945 | Sortase-modified VHH domains and uses thereof | Whitehead Institute for Biomedical Research | 13-
Apr- 12 |
15-
Apr -13 |
5-
Sep -17 |
| US9750798 | Bunyaviruses with segmented glycoprotein precursor genes and methods for generating these viruses | STICHTING WAGENINGEN RESEARCH | 21-
May- 13 |
21-
May -14 |
5-
Sep -17 |
| US9750797 | Sustained release vaccine composition | VIRBAC CORPORATION | 16-
Jun- 04 |
16-
Jun -05 |
5-
Sep -17 |
| US9750690 | Circulation of components during microfluidization and/or homogenization of emulsions | NOVARTIS AG | 3-
Dec- 09 |
5-
Sep -14 |
5-
Sep -17 |
| US9746985 | System and method for detecting, collecting, analyzing, and communicating event-related information | Georgetown University | 25-
Feb- 08 |
20-
Apr -11 |
29-
Aug -17 |
| US9746459 | Antigen presenting cell assay | University of Pittsburgha€”Of the Commonwealth System of Higher Education | 8-
Apr- 10 |
11-
Oct -13 |
29-
Aug -17 |
| US9745306 | 2-((4-amino-3-(3-fluoro-5-hydroxyphenyl)-1H-pyrazolo[3,4- D]pyrimidin-1-yl)methyl)-3-(2-(trifluoromethyl)benzyl) quinazolin- 4(3H)-one derivatives and their use as phosphoinositide 3-kinase inhibitors | Respivert Li mited | 15-
Mar- 13 |
14-
Mar -14 |
29-
Aug -17 |
| US9744231 | Quality control methods for oil-in-water emulsions containing squalene | NOVARTIS AG | 8-
Nov- 06 |
27-
Aug -13 |
29-
Aug -17 |
| US9744229 | Vaccines and immunotherapeutics using IL-28 and compositions and methods of using the same | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | 4-
Apr- 08 |
28-
Apr -14 |
29-
Aug -17 |
| US9744183 | Nucleic acid prodrugs and methods of use thereof | WAVE LIFE SCIENCES LTD. | 6-Jul-
09 |
6-
Jul- 10 |
29-
Aug -17 |
| US9738894 | Short interfering RNA (siRNA) analogues | Roche Innovation Center Copenhagen A/S | 21-
Mar- 03 |
28-
Mar -16 |
22-
Aug -17 |
| US9738624 | Nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 21-
Jun- 13 |
20-
Jun -14 |
22-
Aug -17 |
| US9737618 | Adeno-associated virus (AAV) glades, sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 30-
Sep- 03 |
20-
Jul- 15 |
22-
Aug -17 |
| US9737593 | Carbon nanotube compositions and methods of use thereof | Yale University | 19-
Mar- 08 |
15-
Mar -13 |
22-
Aug -17 |
| US9730997 | Alphavirus vectors for respiratory pathogen vaccines | Novartis Vaccines and Diagnostics, Inc. | 21-
May- 04 |
20-
Aug -14 |
15-
Aug -17 |
| US9730912 | Pharmaceutical compounds | ASTEX THERAPEUTICS LIMITED | 12-
Oct- 06 |
12-
Oct -07 |
15-
Aug -17 |
| US9727810 | Spatially addressable molecular barcoding | Cellular Research, Inc. | 27-
Feb- 15 |
26-
Feb -16 |
8-
Aug -17 |
| US9726607 | Systems and methods for detecting multiple optical signals | GEN-PROBE INCORPORATED | 10-
Mar- 05 |
3-
Mar -14 |
8-
Aug -17 |
| US9725770 | Methods and compositions for identification of source of microbial contamination in a sample | The Regents of the University of California | 6-
Mar- 12 |
6-
Mar -13 |
8-
Aug -17 |
| US9725487 | Compositions and methods for measles virus inhibition | Autoimmune Technologies, LLC | 4-
Nov- 03 |
13-
May -15 |
8-
Aug -17 |
| US9719106 | Tissue preferential codon modified expression cassettes, vectors containing same, and uses thereof | The Trustees of the University of Pennsylvania | 29-
Apr- 13 |
29-
Apr -14 |
1-
Aug -17 |
| US9719083 | Bioagent detection methods | IBIS BIOSCIENCES, INC. | 8-
Mar- 09 |
8-
Mar -10 |
1-
Aug -17 |
| US9718774 | Indole carboxamide derivatives as P2X7 receptor antagonist | IDORSIA PHARMACEUTICALS LTD | 12-
Dec- 12 |
11-
Dec -13 |
1-
Aug -17 |
| US9717755 | Method of treating inflammation | Cytosorbents Corporation | 1-
Apr- 10 |
1-
Apr -11 |
1-
Aug -17 |
| US9717749 | Production of stable non-polyadenylated RNAs | Massachusetts Institute of Technology | 16-
Oct- 12 |
16-
Oct -13 |
1-
Aug -17 |
| US9717732 | Drug combination | VERONA PHARMA PLC | 15-
Mar- 13 |
17-
Mar -14 |
1-
Aug -17 |
| US9714411 | Animal protein-free media for cultivation of cells | Baxalta GmbH | 29-
Oct- 04 |
30-
Nov -15 |
25-
Jul- 17 |
| US9714283 | Compositions and methods for the treatment of immunodeficiency | ADMA BIOLOGICS, INC. | 28-
Oct- 14 |
2-
Jul- 15 |
25-
Jul- 17 |
| US9714226 | Hydrazide containing nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 29-
Jul-11 |
13-
Nov -15 |
25-
Jul- 17 |
| US9713641 | Anti-TIGIT antigen-binding proteins and methods of use thereof | Potenza Therapeutics, Inc. | 13-
Feb- 17 |
13-
Feb -17 |
25-
Jul- 17 |
| US9713606 | Methods for treating pulmonary emphysema using substituted 2- Aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)- amides inhibitors of cathepsin C | Boehringer Ingelheim International GmbH | 14-
Mar- 13 |
1-
Dec -15 |
25-
Jul- 17 |
| US9708375 | Inhibitory polypeptides specific to WNT inhibitors | Amgen Inc. | 15-
Mar- 13 |
14-
Mar -14 |
18-
Jul- 17 |
| US9707278 | Methods of modulating immune responses by modifying Akt3 bioactivity | Augusta University Research Institute, Inc. | 17-
Apr- 14 |
17-
Apr -15 |
18-
Jul- 17 |
| US9701736 | Influenza hemagglutinin-specific monoclonal antibodies for preventing and treating influenza virus infection | New York Blood Center, Inc. | 20-
Oct- 10 |
9-
Oct -14 |
11-
Jul- 17 |
| US9701638 | Therapeutic hydroxyquinolones | Rutgers, The State University of New Jersey | 9-
Nov- 12 |
8-
Nov -13 |
11-
Jul- 17 |
| US9700616 | Arranging interaction and back pressure chambers for microfluidization | NOVARTIS AG | 3-
Dec- 09 |
22-
Mar -16 |
11-
Jul- 17 |
| US9700614 | Intranasal vaccination dosage regimen | Eurocine Vaccines AB | 17-
Dec- 12 |
17-
Dec -13 |
11-
Jul- 17 |
| US9700558 | Drug combination of PDE3/PDE4 inhibitor and muscarinic receptor antagonist | VERONA PHARMA PLC | 15-
Mar- 13 |
17-
Mar -14 |
11-
Jul- 17 |
| US9696247 | Sample fixation and stabilization | RNASSIST LTD. | 1-
Mar- 13 |
28-
Feb -14 |
4-
Jul- 17 |
| US9695445 | Method for production of reprogrammed cell using chromosomally unintegrated virus vector | ID Pharma Co., Ltd. | 16-
Jul-08 |
29-
Jul- 15 |
4-
Jul- 17 |
| US9695135 | Therapeutic catechols | Rutgers, The State University of New Jersey | 12-
May- 14 |
11-
May -15 |
4-
Jul- 17 |
| US9695134 | 3,5-diamino-6-chloro-N-(n-(4-phenylbutyl)carbamimidoyl)pyrazine- 2-carboxamide compounds | Parion Sciences, Inc. | 17-
Dec- 12 |
8-
Jan -15 |
4-
Jul- 17 |
| US9689018 | Mixed cell diagnostic systems for detection of respiratory, herpes and enteric viruses | Diagnostic Hybrids, Inc. | 24-
Apr- 98 |
4-
Aug -14 |
27-
Jun- 17 |
| US9688982 | Methods and compositions for the treatment of cancer or other diseases | CITY OF HOPE | 26-
Jan- 07 |
11-
Oct -13 |
27-
Jun- 17 |
| US9687536 | Methods and compositions for intranasal delivery | SHIN NIPPON BIOMEDICAL LABORATORIES, LTD. | 15-
Apr- 10 |
15-
Apr -11 |
27-
Jun- 17 |
| US9683256 | Biological specimen collection and transport system | Longhorn Vaccines and Diagnostics, LLC | 1-
Oct- 07 |
15-
Dec -15 |
20-
Jun- 17 |
| US9683017 | Inhibitory peptides of viral infection | UNIVERSITY TENNESSEE RESEARCH FOUNDATION | 17-
Jul-14 |
16-
Jul- 15 |
20-
Jun- 17 |
| US9682133 | Disrupted adenovirus-based vaccine against drugs of abuse | CORNELL UNIVERSITY | 17-
Mar- 10 |
17-
Mar -11 |
20-
Jun- 17 |
| US9677089 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
30-
Mar -16 |
13-
Jun- 17 |
| US9676867 | Chimeric T cell receptor comprising carbonic anhydrase IX (G250) antibody | Dana-Farber Cancer Institute Inc. | 2-
Dec- 05 |
8-
May -13 |
13-
Jun- 17 |
| US9676857 | Soluble engineered monomeric Fc | The United States of America, as represented by the Secretary, Department of Health and Human Services | 16-
Mar- 12 |
14-
Mar -13 |
13-
Jun- 17 |
| US9676727 | Myxovirus therapeutics, compounds, and uses related thereto | Children’s Healthcare of Atlanta, Inc. | 24-
Oct- 11 |
7-
Jul- 16 |
13-
Jun- 17 |
| US9675550 | Methods for inducing an immune response via buccal and/or sublingual administration of a vaccine | BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM | 26-
Jul-10 |
25-
Jan -13 |
13-
Jun- 17 |
| US9670507 | Directed evolution and in vivo panning of virus vectors | The University of North Carolina at Chapel Hill | CO 1 1 | 28-
Jun -16 |
6-
Jun- 17 |
| US9670166 | Substituted bicyclic dihydropyrimidinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 6-
Feb- 13 |
5-
Aug -16 |
6-
Jun- 17 |
| US9669092 | Antagonism of the VIP signaling pathway | Emory University | 2-
Feb- 11 |
31-
Jan -12 |
6-
Jun- 17 |
| US9669089 | Nucleic acid comprising or coding for a histone stem-loop and a poly(A) sequence or a polyadenylation signal for increasing the expression of an encoded pathogenic antigen | CureVac AG | 15-
Feb- 12 |
15-
Feb -13 |
6-
Jun- 17 |
| US9669088 | Vaccination with multiple clades of H5 influenza A virus | Seqirus UK Limited | 26-
Nov- 07 |
25-
Nov -08 |
6-
Jun- 17 |
| US9661856 | Synergy of plant antimicrobials with silver | The Arizona Board of Regents on Behalf of The University of Arizona | 24-
Aug- 12 |
26-
Aug -13 |
30-
May -17 |
| US9657278 | Methods to produce bunyavirus replicon particles | Stichting Dienst Landbouwkundig Onderzoek | 20-
Sep- 10 |
10-
Jul- 15 |
23-
May -17 |
| US9657076 | GM-CSF and IL-4 conjugates, compositions, and methods related thereto | Children’s Healthcare of Atlanta, Inc. | 23-
Oct- 12 |
23-
Oct -13 |
23-
May -17 |
| US9657048 | Enantiomers of the 1a€2,6a€2-isomer of neplanocin A | Auburn University | 4-
Aug- 14 |
4-
Aug -15 |
23-
May -17 |
| US9657015 | Substituted bicyclic dihydropyrimidinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 31-
Jul-14 |
27-
Jul- 15 |
23-
May -17 |
| US9655896 | Chemically and metabolically stable dipeptide possessing potent sodium channel blocker activity | PARION SCIENCES, INC. | 27-
Jun- 11 |
12-
Jan -15 |
23-
May -17 |
| US9655845 | Oil-in-water emulsions that contain nucleic acids | GlaxoSmithKline Biologicals, S.A. | 6-Jul-
11 |
6-
Jul- 12 |
23-
May -17 |
| US9655367 | Disinfecting composition and wipes with reduced contact time | LONZA, INC. | 6-
Nov- 13 |
4-
Nov -14 |
23-
May -17 |
| US9651543 | Malaria antigen screening method | The United States of America as Represented by the Secretary of the Navy | 31-
Aug- 05 |
19-
Apr -13 |
16-
May -17 |
| US9650685 | Selective detection of human rhinovirus | The United States of America, as represented by the Secretary, Department of Health and Human Services | 5-
Dec- 08 |
15-
Dec -14 |
16-
May -17 |
| US9650649 | LCMV-GP-VSV-pseudotyped vectors and tumor-infiltrating virus- producing cells for the therapy of tumors | VIRATHERAPEUTICS GMBH | 8-
Oct- 08 |
8-
Oct -09 |
16-
May -17 |
| US9649324 | Use of tylvalosin as antiviral agent | CAMBRIDGE UNIVERSITY TECHNICAL SERVICES | 13-
Jul-06 |
8-
Jun -15 |
16-
May -17 |
| US9649309 | Therapeutic uses of selected pyrimidine compounds with anti-Mer tyrosine kinase activity | The University of North Carolina at Chapel Hill | 11-
Apr- 14 |
3-
Apr -15 |
16-
May -17 |
| US9644180 | Synthetic membrane-receiver complexes | RUBIUS THERAPEUTICS, INC. | 18-
Nov- 13 |
12-
Jun -15 |
9-
May -17 |
| US9642876 | Method of preventing or treating sinusitis with oxidative reductive potential water solution | SONOMA PHARMACEUTICALS, INC. | 30-
Dec- 03 |
27-
Oct -15 |
9-
May -17 |
| US9642873 | Combinations of TGFÎ2 and COX-2 inhibitors and methods for their therapeutic application | Sirnaomics, Inc. | 4-
May- 10 |
4-
May -11 |
9-
May -17 |
| US9637524 | Proteolysis-resistant capsid of chimeric hepatitis E virus as an oral delivery vector | The Regents of the University of California | 27-
Feb- 09 |
5-
Nov -14 |
2-
May -17 |
| US9637491 | Pyrazolo[4,3-D]pyrimidines as kinase inhibitors | ORIGENIS GMBH | 19-
Oct- 12 |
18-
Oct -13 |
2-
May -17 |
| US9636410 | Cationic oil-in-water emulsions | GLAXOSMITHKLINE BIOLOGICALS SA | 6-Jul-
11 |
6-
Jul- 12 |
2-
May -17 |
| US9636397 | Adjuvant compositions and related methods | Vaxliant, LLC | 24-
Mar- 15 |
24-
Mar -16 |
2-
May -17 |
| US9636370 | AAV vectors targeted to oligodendrocytes | The University of North Carolina at Chapel Hill | 28-
Sep- 12 |
27-
Sep -13 |
2-
May -17 |
| US9629907 | Compositions for and methods of inducing mucosal immune responses | The Trustees of the University of Pennsylvania | 19-
Nov- 04 |
28-
Oct -11 |
25-
Apr- 17 |
| US9624173 | Heterocyclic modulators of lipid synthesis | 3-V Biosciences, Inc. | 8-
Mar- 11 |
19-
Mar -15 |
18-
Apr- 17 |
| US9623040 | Immunomodulation by controlling expression levels of microRNAs in dendritic cells | The Board of Trustees of the Leland Stanford Junior University | 14-
Jul-14 |
10-
Jul- 15 |
18-
Apr- 17 |
| US9618508 | Flow cytometry analysis of materials adsorbed to metal salts | GlaxoSmithKline Biologicals SA | 14-
Dec- 10 |
14-
Dec -11 |
11-
Apr- 17 |
| US9618476 | System and method for electronic biological sample analysis | NANOMEDICAL DIAGNOSTICS, INC. | 28-
Apr- 14 |
28-
Apr -14 |
11-
Apr- 17 |
| US9618429 | Polymer stabilization of chromogen solutions | Ventana Medical Systems, Inc. | 23-
Jan- 12 |
18-
Jan -13 |
11-
Apr- 17 |
| US9611481 | Chimeric polynucleotides and polypeptides enabling the secretion of a polypeptide of interest in combination with exosomes and uses thereof | UNIVERSITE DE MONTPELLIER | 24-
Sep- 09 |
23-
Sep -10 |
4-
Apr- 17 |
| US9611474 | Double-stranded oligonucleotide molecules to DDIT4 and methods of use thereof | QUARK PHARMACEUTICALS, INC. | 12-
Sep- 12 |
12-
Sep -13 |
4-
Apr- 17 |
| US9605276 | Replication defective adenovirus vector in vaccination | Etubics Corporation | 24-
Aug- 12 |
15-
Mar -13 |
28-
Mar -17 |
| US9603864 | Substituted nucleosides, nucleotides and analogs thereof | Alios BioPharma, Inc. | 24-
Jun- 14 |
22-
Jun -15 |
28-
Mar -17 |
| US9603850 | MerTK-specific pyrazolopyrimidine compounds | The University of North Carolina at Chapel Hill | 11-
Apr- 14 |
3-
Apr -15 |
28-
Mar -17 |
| US9599606 | ADP-ribose detection reagents | The Board of Regents of the University of Texas System | 10-
Jun- 14 |
9-
Jun -15 |
21-
Mar -17 |
| US9598459 | Pharmaceutical compositions and methods | Pop Test Oncology LLC | 3-
Aug- 15 |
28-
Jul- 16 |
21-
Mar -17 |
| US9597333 | Benzazepine dicarboxamide compounds | Hoffmann-La Roche Inc. | 6-
Mar- 15 |
28-
Sep -16 |
21-
Mar -17 |
| US9593334 | Use of the chromosome 19 microRNA cluster (C19MC) for treating viral disease and promoting authophagy | University of Pittsburgh—Of the Commonwealth System of Higher Education | 7-
Mar- 12 |
6-
Mar -13 |
14-
Mar -17 |
| US9593331 | Double-stranded nucleic acid molecule for gene expression control | Osaka City University | 2-
Nov- 11 |
1-
Nov -12 |
14-
Mar -17 |
| US9593084 | Chloro-pyrazine carboxamide derivatives with epithelial sodium channel blocking activity | Parion Sciences, Inc. | 17-
Dec- 12 |
13-
Dec -13 |
14-
Mar -17 |
| US9592284 | Immunization regimen with E4-deleted adenovirus prime and E1- deleted adenovirus boost | The Trustees of the University of Pennsylvania | 28-
Apr- 04 |
27-
Apr -05 |
14-
Mar -17 |
| US9592277 | Compositions with modified nucleases targeted to viral nucleic acids and methods of use for prevention and treatment of viral diseases | Avirid, Inc. | 14-
Apr- 04 |
14-
Apr -05 |
14-
Mar -17 |
| US9588069 | Methods for performing thermal melt analysis | GEN-PROBE INCORPORATED | 31-
Jul-12 |
31-
Jul- 13 |
7-
Mar -17 |
| US9587250 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | Trustees of the University of Pennsylvania | 17-
Dec- 01 |
16-
Jan -15 |
7-
Mar -17 |
| US9586998 | Methods of propagating monkey adenoviral vectors | GenVec, Inc. | 9-
Nov- 09 |
4-
Aug -15 |
7-
Mar -17 |
| US9586911 | Arylalkyl- and aryloxyalkyl-substituted epthelial sodium channel blocking compounds | Parion Sciences, Inc. | 13-
Dec- 13 |
19-
Dec -14 |
7-
Mar -17 |
| US9586910 | 3,5-diamino-6-chloro-N-(N-(4-(4-(2-(hexyl(2,3,4,5,6-
pentahydroxyhexyl)amino)ethoxy)phenyl)butyl)carbamimidoyl)pyra zine-2-carboxamide |
Parion Sciences, Inc. | 27-
Jun- 11 |
18-
Dec -13 |
7-
Mar -17 |
| US9585968 | Hydrazino 1H-imidazoquinolin-4-amines and conjugates made therefrom | 3M Innovative Properties Company | 3-
Jun- 11 |
14-
Aug -15 |
7-
Mar -17 |
| US9585953 | Immunogenic compositions in particulate form and methods for producing the same | MUCOSIS B.V. | 22-
Mar- 11 |
22-
Mar -12 |
7-
Mar -17 |
| US9585874 | Nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 9-
May- 12 |
6-
Jan -16 |
7-
Mar -17 |
| US9585849 | Broad spectrum antiviral and methods of use | The Burlington HC Research Group, Inc. | 17-
Apr- 06 |
17-
Apr -07 |
7-
Mar -17 |
| US9580474 | Polyionic papilloma virus-like particle (VLP) vaccines | THE JOHNS HOPKINS UNIVERSITY | 8-
Sep- 10 |
8-
Sep -11 |
28-
Feb -17 |
| US9580468 | Methods and reagents for efficient and targeted delivery of therapeutic molecules to CXCR4 cells | CENTRE DE INVESTIGACION BIOMEDICA EN RED BIOINGENIERA BIOMATERIALS Y NANOMEDICINA (CIBER BBN) | 13-
Jan- 11 |
13-
Jan -12 |
28-
Feb -17 |
| US9580429 | Pyrrolo[3,2-D]pyrimidin-4-one derivatives and their use in therapy | AstraZeneca AB | 6-
Dec- 04 |
15-
Sep -14 |
28-
Feb -17 |
| US9574189 | Enzymatic encoding methods for efficient synthesis of large libraries | Nuevolution A/S | 1-
Dec- 05 |
1-
Dec -06 |
21-
Feb -17 |
| US9574181 | Influenza virus reassortment method | Seqirus UK Limited | 21-
May- 10 |
24-
Oct -14 |
21-
Feb -17 |
| US9573955 | Compounds | Chiese Farmaceutici S.p.A. | 16-
Dec- 13 |
16-
Dec -14 |
21-
Feb -17 |
| US9573938 | Therapeutic hydroxypyridinones, hydroxypyrimidinones and hydroxypyridazinones | Rutgers, The State University of New Jersey | 11-
Sep- 12 |
11-
Sep -13 |
21-
Feb -17 |
| US9572899 | Compositions for enhancing transport of molecules into cells | AVI BIOPHARMA, INC. | 29-
Apr- 03 |
5-
Nov -08 |
21-
Feb -17 |
| US9572864 | Compositions and uses of lectins | Emory University | 12-
Feb- 10 |
11-
Dec -15 |
21-
Feb -17 |
| US9572823 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
5-
Apr -16 |
21-
Feb -17 |
| US9567336 | Conjugated TLR7 and/or TLR8 and TLR2 agonists | INVIVOGEN | 19-
Nov- 12 |
15-
Mar -13 |
14-
Feb -17 |
| US9566326 | Adjuvanted influenza vaccines for pediatric use | Seqirus UK Limited | 22-
Feb- 08 |
21-
Jun -13 |
14-
Feb -17 |
| US9566291 | Nutritional composition comprising indigestible oligosaccharides | N.V. Nutricia | 24-
Aug- 04 |
24-
Aug -05 |
14-
Feb -17 |
| US9566290 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
20-
Apr -16 |
14-
Feb -17 |
| US9566289 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
17-
Feb -16 |
14-
Feb -17 |
| US9565857 | Antimicrobial solutions | Board of Regents, The University of Texas System | 10-
Sep- 10 |
9-
Sep -11 |
14-
Feb -17 |
| US9562110 | Bispecific antibody | Wuhan YZY Biopharma Co., Ltd. | 21-
Nov- 12 |
21-
Nov -12 |
7-
Feb -17 |
| US9561263 | Treatment of inflammatory illnesses with ACE2 | Apeiron Biologics AG | 18-
Dec- 07 |
18-
Dec -08 |
7-
Feb -17 |
| US9556237 | Antiviral rift valley fever virus peptides and methods of use | The United States of America, as represented by the Secretary of the Army, on behalf of the U.S. Army Medical Research Institute of Infectious Diseases | 6-
Dec- 12 |
4-
Jun -15 |
31-
Jan- 17 |
| US9556229 | Modification of peptides using a bis(thioether)arylbridge approach | The Regents of the University of California | 18-
May- 12 |
17-
May -13 |
31-
Jan- 17 |
| US9556184 | Phosphoinositide 3-kinase inhibitors | Respivert, Ltd. | 15-
Mar- 13 |
4-
Dec -15 |
31-
Jan- 17 |
| US9556117 | Indole carboxamide derivatives as P2X7 receptor antagonists | ACTELION PHARMACEUTICALS LTD. | 18-
Dec- 12 |
17-
Dec -13 |
31-
Jan- 17 |
| US9555031 | Therapeutic uses of selected pyrrolopyrimidine compounds with anti-mer tyrosine kinase activity | The University of North Carolina at Chapel Hill | 11-
Apr- 14 |
3-
Apr -15 |
31-
Jan- 17 |
| US9555030 | Therapeutic uses of selected pyrazolopyrimidine compounds with anti-Mer tyrosine kinase activity | The University of North Carolina at Chapel Hill | 11-
Apr- 14 |
3-
Apr -15 |
31-
Jan- 17 |
| US9550773 | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines | 3M Innovative Properties Company | 18-
Jun- 04 |
13-
Apr -15 |
24-
Jan- 17 |
| US9550757 | Nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 5-
Mar- 10 |
28-
Jun -13 |
24-
Jan- 17 |
| US9549949 | Antiviral agent | NBC MESHTEC, INC. | 3-
Sep- 08 |
31-
Aug -09 |
24-
Jan- 17 |
| US9549938 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
11-
Mar -16 |
24-
Jan- 17 |
| US9546371 | Chimeric polynucleotides and polypeptides enabling secretion of a polypeptide of interest in association with exosomes and use thereof for the production of immunogenic compositions | CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE | 18-
Mar- 08 |
18-
Mar -09 |
17-
Jan- 17 |
| US9546184 | Alkyloxy substituted thiazoloquinolines and thiazolonaphthyridines | 3M INNOVATIVE PROPERTIES COMPANY | 13-
Aug- 08 |
8-
Jun -15 |
17-
Jan- 17 |
| US9546150 | Substituted quinazolin-4-ones for inhibiting ubiquitin specific protease 7 | HYBRIGENICS SA | 2-
Sep- 11 |
29-
Aug -12 |
17-
Jan- 17 |
| US9545440 | Methods for preparing squalene | NOVARTIS AG | 12-
May- 10 |
23-
Oct -15 |
17-
Jan- 17 |
| US9540373 | Substituted spirocycles | Boehringer Ingelheim International GmbH | 12-
Sep- 14 |
10-
Sep -15 |
10-
Jan- 17 |
| US9539321 | HMGBl-derived peptides enhance immune response to antigens | The Regents of the University of California | 27-
Jul-10 |
9-
Mar -15 |
10-
Jan- 17 |
| US9539217 | Nanoparticle compositions | Allertein Therapeutics, LLC | 3-
Apr- 13 |
3-
Apr -14 |
10-
Jan- 17 |
| US9533978 | Pyrimidine derivatives and their use in the treatment of cancer and further diseases | Sumitomo Dainippon Pharma Co., Ltd | 21-
May- 09 |
26-
Aug -14 |
3-
Jan- 17 |
| US9533037 | Methods for designing and preparing vaccines comprising directed sequence polymer compositions via the directed expansion of epitopes | Declion Holdings LLC | 16-
Oct- 07 |
16-
Oct -08 |
3-
Jan- 17 |
| US9529974 | System and method for detecting, collecting, analyzing, and communicating event-related information | Georgetown University | 25-
Feb- 08 |
27-
Jul- 11 |
27-
Dec -16 |
| US9527903 | Engineered antibody constant domain molecules | The United States of America, as represent by the Secretary, Department of Health and Human Services | 31-
Jan- 08 |
1-
Oct -13 |
27-
Dec -16 |
| US9526803 | Diagnostic chewing gum for pathogens | Julius-Maximilians-Universitaet Wuerzburg | 8-
Mar- 12 |
8-
Mar -13 |
27-
Dec -16 |
| US9526700 | Composition for inactivating an enveloped virus | VIROBLOCK SA | 19-
May- 06 |
17-
Nov -14 |
27-
Dec -16 |
| US9522962 | Peptides, conjugates and method for increasing immunogenicity of a vaccine | Academisch Ziekenhuis Leiden h.o.d.n. LUMC | 15-
Mar- 10 |
15-
Mar -11 |
20-
Dec -16 |
| US9522894 | Certain (2S)-N-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2- carboxamides as dipeptidyl peptidase 1 inhibitors | AstraZeneca AB | 24-
Jan- 14 |
21-
Jan -15 |
20-
Dec -16 |
| US9522171 | EV576 for use in the treatment of viral infections of the respiratory tra ct | Volution Immuno Pharmaceuticals SA | 8-
Jan- 10 |
2-
Jan -15 |
20-
Dec -16 |
| US9518093 | Topical formulation of arginine-rich cyclic antimicrobial peptides | NOVABIOTICS LIMITED | 24-
Feb- 06 |
9-
Nov -15 |
13-
Dec -16 |
| US9518083 | Gadd45beta targeting agents | Imperial Innovations Limited | 22-
Oct- 09 |
10-
Feb -15 |
13-
Dec -16 |
| US9517263 | Benzonaphthyridine-containing vaccines | GlaxoSmithKline Biologicals SA | 10-
Jun- 09 |
10-
Jun -10 |
13-
Dec -16 |
| US9517205 | Soluble needle arrays for delivery of influenza vaccines | Seqirus UK Limited | 20-
Aug- 10 |
19-
Aug -11 |
13-
Dec -16 |
| US9512471 | Methods and kits for detecting human papillomavirus | DIACARTA Inc | 30-
Jun- 10 |
30-
Jun -10 |
6-
Dec -16 |
| US9512443 | Recombinant expression of multiprotein complexes using polygenes | ETH ZURICH | 8-
Nov- 05 |
6-
Nov -06 |
6-
Dec -16 |
| US9512181 | Fusion proteins of ciliate granule lattice proteins, granular protein particles thereof, and uses therefor | Tetragenetics, Inc. | 27-
May- 11 |
29-
May -12 |
6-
Dec -16 |
| US9511070 | Heterocyclyl carboxamides for treating viral diseases | NovaDrug, LLC | 31-
Aug- 12 |
30-
Aug -13 |
6-
Dec -16 |
| US9506063 | SiRNA compositions and methods for treatment of HPV and other infections | Sirnaomics, Inc. | 29-
Jul-10 |
29-
Jan -13 |
29-
Nov -16 |
| US9504747 | Lipids and lipid compositions for the delivery of active agents | Novartis AG | 8-
Mar- 13 |
7-
Mar -14 |
29-
Nov -16 |
| US9504673 | Agent for the prophylaxis and treatment of highly pathogenic infectious diseases | LTD “Valenta-Intellekt†| 21-
May- 09 |
10-
May -10 |
29-
Nov -16 |
| US9504255 | Physical antimicrobial method | NMS TECHNOLOGIES CO., LTD. | 1-
Aug- 12 |
16-
Jul- 13 |
29-
Nov -16 |
| US9499799 | Cells and methodology to generate non-segmented negative-strand RNA viruses | Centre National De La Recherche Scientifique | 22-
Dec- 06 |
15-
Oct -13 |
22-
Nov -16 |
| US9499535 | Kinase inhibitors | ORIGENIS GMBH | 21-
Apr- 11 |
23-
Apr -12 |
22-
Nov -16 |
| US9499489 | Myxovirus therapeutics, compounds, and uses related thereto | Children’s Healthcare of Atlanta, Inc. | 24-
Oct- 11 |
24-
Oct -12 |
22-
Nov -16 |
| US9498548 | Method of using oxidative reductive potential water solution in dental applications | Oculus Innovative Sciences, Inc. | 2-
May- 05 |
2-
May -06 |
22-
Nov -16 |
| US9498544 | Genetically modified human umbilical cord perivascular cells for prophylaxis against or treatment of biological or chemical agents | Tissue Regeneration Therapeutics Inc. | 21-
Apr- 08 |
13-
Mar -15 |
22-
Nov -16 |
| US9498527 | Vaccine composition | NITTO DENKO CORPORATION | 4-
Apr- 12 |
3-
Apr -13 |
22-
Nov -16 |
| US9494571 | Methods of testing for intracellular pathogens | Novartis AG | 8-
Mar- 10 |
7-
Mar -11 |
15-
Nov -16 |
| US9493788 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
16-
Jan -15 |
15-
Nov -16 |
| US9493572 | GITR antibodies and methods of inducing or enhancing an immune response | GITR, Inc. | 25-
Mar- 05 |
23-
Mar -15 |
15-
Nov -16 |
| US9493518 | Compositions and methods for treating clostridium difficile- associated diseases | National Health Research Institutes | 14-
Mar- 13 |
13-
Mar -14 |
15-
Nov -16 |
| US9492528 | Influenza virus-like particles (VLPS) comprising hemagglutinin | MEDICAGO INC. | 13-
Jul-07 |
2-
Jul- 09 |
15-
Nov -16 |
| US9492413 | Use of salt of an acetylsalicylic acid for the treatment of viral infections | Ventaleon GMBH | 14-
Jan- 08 |
14-
Jan -09 |
15-
Nov -16 |
| US9489495 | System and method for detecting, collecting, analyzing, and communicating event-related information | GEORGETOWN UNIVERSITY | 25-
Feb- 08 |
28-
Aug -08 |
8-
Nov -16 |
| US9487838 | Oligonucleotide probe for the detection of adenovirus | QIAGEN HAMBURG GMBH | 28-
Dec- 10 |
28-
Dec -10 |
8-
Nov -16 |
| US9487837 | Exosome-mediated diagnosis of hepatitis virus infections and diseases | MOREHOUSE SCHOOL OF MEDICINE | 6-
Oct- 08 |
21-
Jan -14 |
8-
Nov -16 |
| US9487778 | Oligonucleotide modulators of the toll-like receptor pathway | QUARK PHARMACEUTICALS, INC. | 3-
Mar- 11 |
1-
Mar -12 |
8-
Nov -16 |
| US9487749 | Use of methylsulfonylmethane (MSM) to modulate microbial activity | Biogenic Innovations, LLC | 30-
Oct- 09 |
12-
Aug -14 |
8-
Nov -16 |
| US9487528 | Compounds | Chiesi Farmaceutici S.p.A. | 9-
Jun- 14 |
5-
Jun -15 |
8-
Nov -16 |
| US9486479 | Antimicrobial solutions containing dichloride monoxide and methods of making and using the same | Oculus Innovative Sciences, Inc. | 13-
Mar- 07 |
21-
Jul- 14 |
8-
Nov -16 |
| US9481912 | Compositions and methods for detecting and identifying nucleic acid sequences in biological samples | Longhorn Vaccines and Diagnostics, LLC | 12-
Sep- 06 |
8-
Oct -13 |
1-
Nov -16 |
| US9481724 | hDC-sign binding peptides | Sloan-Kettering Institute for Cancer Research | 19-
Dec- 11 |
10-
Dec -12 |
1-
Nov -16 |
| US9481630 | Ingenane-type diterpene compound, and pharmaceutical composition for treating or preventing viral infectious diseases containing same | KOREA RESEARCH INSTITUTE OF BIOSCIENCE AND BIOTECHNOLOGY | 19-
Oct- 11 |
28-
Sep -12 |
1-
Nov -16 |
| US9476090 | Signal propagation biomolecules, devices and methods | STC.UNM | 21-
May- 13 |
21-
May -14 |
25-
Oct- 16 |
| US9476032 | Attenuated viruses useful for vaccines | The Research Foundation for The State University of New York | 30-
Mar- 07 |
31-
Mar -08 |
25-
Oct- 16 |
| US9475872 | Nucleic acid molecules encoding moonoclonal antibodies speceific for IL17F | ImmunoQure AG | 28-
Dec- 11 |
2-
Jan -13 |
25-
Oct- 16 |
| US9475862 | Neutralizing GP41 antibodies and their use | The United States of America, as represented by the Secretary, Department of Health and Human Services | 7-
Nov- 11 |
7-
Nov -12 |
25-
Oct- 16 |
| US9475832 | Phosphonates with reduced toxicity for treatment of viral infections | The Regents of the University of California | 14-
Apr- 10 |
29-
Sep -14 |
25-
Oct- 16 |
| US9475804 | Heterobifunctional linkers with polyethylene glycol segments and immune response modifier conjugates made therefrom | 3M Innovative Properties Company | 3-
Jun- 11 |
1-
Jun -12 |
25-
Oct- 16 |
| US9475779 | Substituted bicyclic dihydropyrimidinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 31-
Jul-14 |
27-
Jul- 15 |
25-
Oct- 16 |
| US9475775 | Benzazepine dicarboxamide compounds | Hoffmann-La Roche Inc. | 6-
Mar- 15 |
4-
Mar -16 |
25-
Oct- 16 |
| US9474844 | Methods for pathogen inactivation in blood using UV irradiation while minimizing heat transfer thereto | Hemalux LLC | 22-
Oct- 14 |
29-
Dec -15 |
25-
Oct- 16 |
| US9474759 | Broad-spectrum antivirals against 3C or 3C-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses | Kansas State University Research Foundation | 27-
Sep- 11 |
27-
Sep -12 |
25-
Oct- 16 |
| US9469876 | Circulating biomarkers for metastatic prostate cancer | Caris Life Sciences Switzerland Holdings GmbH | 6-
Apr- 10 |
6-
Apr -11 |
18-
Oct- 16 |
| US9464276 | Highly efficient influenza matrix (M1) proteins | Novavax, Inc. | 11-
Jul-03 |
23-
Feb -15 |
11-
Oct- 16 |
| US9464123 | Peptides having activity of inhibiting infections of respiratory viruses and use of the same | XIANGXUE GROUP (HONG KONG) COMPANY LIMITED | 9-
May- 13 |
21-
Feb -14 |
11-
Oct- 16 |
| US9463240 | Arranging interaction and back pressure chambers for microfluidization | NOVARTIS AG | 3-
Dec- 09 |
11-
Jul- 14 |
11-
Oct- 16 |
| US9459247 | Quantitative measurement of nano/micro particle endocytosis with cell mass spectrometry | Academia Sinica | 29-
Mar- 10 |
29-
Mar -11 |
4-
Oct- 16 |
| US9459233 | Amperometric gas sensor | Steris Corporation | 25-
Jun- 12 |
26-
Feb -13 |
4-
Oct- 16 |
| US9458492 | Methods and cells for identifying RIG-I pathway regulators | Kineta, Inc. | 25-
Feb- 11 |
23-
Feb -12 |
4-
Oct- 16 |
| US9458470 | Recombinant influenza virus-like particles (VLPs) produced in transgenic plants expressing hemagglutinin | MEDICAGO INC. | 21-
Jan- 08 |
23-
Jan -13 |
4-
Oct- 16 |
| US9458184 | Compositions of TLR7 and/or TLR8 agonists conjugated to lipids | INVIVOGEN | 15-
Jun- 12 |
15-
Mar -13 |
4-
Oct- 16 |
| US9458113 | Substituted bicyclic dihydropyrimidinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 31-
Jul-14 |
27-
Jul- 15 |
4-
Oct- 16 |
| US9457074 | Compositions and methods for treating and preventing porcine reproductive and respiratory syndrome | Ohio State Innovation Foundation | 24-
Apr- 12 |
23-
Feb -15 |
4-
Oct- 16 |
| US9453043 | Nucleic acid chemical modifications | ALNYLAM PHARMACEUTICALS, INC. | 2-
Mar- 09 |
22-
Jan -15 |
27-
Sep -16 |
| US9452973 | Modulators of the relaxin receptor 1 | THE FLORIDA INTERNATIONAL UNIVERSITY BOARD OF TRUSTEES | 4-
May- 12 |
15-
Mar -13 |
27-
Sep -16 |
| US9452210 | Influenza virus-like particles (VLPS) comprising hemagglutinin produced within a plant | MEDICAGO INC. | 13-
Jul-07 |
4-
Jan -13 |
27-
Sep -16 |
| US9447462 | Methods for concurrent identification and quantification of an unknown bioagent | IBIS BIOSCIENCES, INC. | 18-
Feb- 04 |
27-
Jan -14 |
20-
Sep -16 |
| US9447132 | Highly active nucleoside derivative for the treatment of HCV | Achillion Pharmaceuticals, Inc. | 12-
Apr- 13 |
14-
Apr -14 |
20-
Sep -16 |
| US9447097 | 4-amino-imidazoquinoline compounds | Hoffmann-La Roche Inc. | 22-
Apr- 14 |
7-
Apr -16 |
20-
Sep -16 |
| US9446062 | Methods of treating ischemia-reperfusion injury with siRNAs | Quark Pharmaceuticals, Inc. | 25-
Oct- 06 |
8-
Jan -15 |
20-
Sep -16 |
| US9442107 | Antibody-nanoparticle conjugates and methods for making and using such conjugates | Ventana Medical Systems, Inc. | 27-
Apr- 10 |
21-
Apr -15 |
13-
Sep -16 |
| US9441247 | TC-83-derived alphavirus vectors, particles and methods | ALPHAVAX, INC. | 18-
May- 04 |
6-
Jul- 15 |
13-
Sep -16 |
| US9440960 | Substituted oxetanes and their use as inhibitors of cathepsin C | Boehringer Ingelheim International GmbH | 1-
Aug- 14 |
31-
Jul- 15 |
13-
Sep -16 |
| US9440930 | Substituted bicyclic dihydropyrimidinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 31-
Jul-14 |
27-
Jul- 15 |
13-
Sep -16 |
| US9435795 | Enhanced deposition of chromogens utilizing pyrimidine analogs | Ventana Medical Systems, Inc. | 30-
Dec- 10 |
6-
Oct -14 |
6-
Sep -16 |
| US9435000 | Primate T-lymphotropic viruses | Johns Hopkins University | 21-
Feb- 05 |
20-
Sep -13 |
6-
Sep -16 |
| US9434997 | Methods, compounds and systems for detecting a microorganism in a sample | Lawrence Livermore National Security, LLC | 24-
Aug- 07 |
21-
Aug -08 |
6-
Sep -16 |
| US9434769 | Peptide compositions and methods for inhibiting herpesvirus infection | The Administrators of the Tulane Educational Fund | 30-
Oct- 09 |
9-
Jul- 14 |
6-
Sep -16 |
| US9433672 | Compositions and methods for activating innate and allergic immunity | ID Biomedical Corporation of Quebec | 26-
Apr- 12 |
28-
Apr -14 |
6-
Sep -16 |
| US9430610 | Re-sequencing pathogen microarray | The United States of America, as represented by the Secretary of the Navy | 2-Jul-
04 |
10-
Apr -08 |
30-
Aug -16 |
| US9428739 | Norovirus and Sapovirus antigens | NOVARTIS VACCINES AND DIAGNOSTICS, INC. | 24-
Mar- 01 |
27-
Nov -12 |
30-
Aug -16 |
| US9428574 | Polypeptides and uses thereof for treatment of autoimmune disorders and infection | COMPUGEN LTD. | 30-
Jun- 11 |
1-
Jul- 12 |
30-
Aug -16 |
| US9428571 | Antibodies and processes for preparing the same | TAIGA BIOTECHNOLOGIES, INC. | 16-
May- 08 |
18-
Mar -15 |
30-
Aug -16 |
| US9428490 | Nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 29-
Jul-11 |
26-
Jul- 12 |
30-
Aug -16 |
| US9428439 | Hydrobenzamide derivatives as inhibitors of Hsp90 | ASTEX THERAPEUTICS LTD. | 12-
Oct- 06 |
7-
Jan -14 |
30-
Aug -16 |
| US9426989 | Organic peroxide compounds for microorganism inactivation | NOVARTIS AG | 6-
May- 10 |
6-
May -11 |
30-
Aug -16 |
| US9422367 | Antigenic GM-CSF peptides and antibodies to GM-CSF | Morphotek, Inc. | 7-
Feb- 06 |
26-
Nov -13 |
23-
Aug -16 |
| US9421254 | Immunostimulatory combinations of TLR ligands and methods of use | The United States of America, as represented by the Secretary, Department of Health and Human Services | 24-
Sep- 07 |
24-
Sep -08 |
23-
Aug -16 |
| US9416416 | Biological specimen collection/transport compositions and methods | Longhorn Vaccines and Diagnostics, LLC | 1-
Oct- 07 |
16-
Dec -11 |
16-
Aug -16 |
| US9416409 | Capture primers and capture sequence linked solid supports for molecular diagnostic tests | IBIS BIOSCIENCES, INC. | 31-
Jul-09 |
30-
Jul- 10 |
16-
Aug -16 |
| US9416396 | Covalently linked thermostable kinase for decontamination process validation | The Secretary of State for Health | 20-
Feb- 08 |
18-
Feb -09 |
16-
Aug -16 |
| US9415392 | Slip chip device and methods | The University of Chicago | 24-
Mar- 09 |
23-
Mar -10 |
16-
Aug -16 |
| US9415087 | Compositions and methods for treating coronavirus infection | Ludwig-Maximilians-Universitaet Muenchen | 11-
Mar- 14 |
8-
May -15 |
16-
Aug -16 |
| US9415033 | Esters of short chains fatty acids for use in the treatment of immunogenic disorders | PROPONENT BIOTECH GMBH | 3-
Oct- 12 |
3-
Oct -13 |
16-
Aug -16 |
| US9409987 | Polypeptides and polynucleotides, and uses thereof for treatment of immune related disorders and cancer | COMPUGEN LTD | 15-
Apr- 11 |
16-
Apr -12 |
9-
Aug -16 |
| US9409917 | Heterocyclic amide derivatives as P2X7 receptor antagonists | ACTELION PHARMACEUTICALS LTD. | 20-
Jan- 12 |
18-
Jan -13 |
9-
Aug -16 |
| US9409870 | Compounds | CHIESI FARMACEUTICI S.p.A. | 15-
Dec- 14 |
27-
Nov -15 |
9-
Aug -16 |
| US9408908 | Combination adjuvant formulation | Not Available | 16-
Oct- 08 |
15-
Feb -13 |
9-
Aug -16 |
| US9408907 | Homogenous suspension of immunopotentiating compounds and uses thereof | GlaxoSmithKline Biologicals SA | 15-
Dec- 09 |
15-
Dec -10 |
9-
Aug -16 |
| US9404160 | Methods for the detection of microorganisms | Becton, Dickinson and Company | 22-
Dec- 09 |
21-
Dec -10 |
2-
Aug -16 |
| US9403868 | Crystalline tripeptide epoxy ketone protease inhibitors | Onyx Therapeutics, Inc. | 20-
Mar- 09 |
5-
Mar -15 |
2-
Aug -16 |
| US9402921 | Directed evolution and in vitro panning of virus vectors | The University of North Carolina at Chapel Hill | 30-
Apr- 08 |
22-
Oct -14 |
2-
Aug -16 |
| US9402878 | Depsipeptide and uses thereof | NovoBiotic Pharmaceuticals, LLC | 3-
Dec- 12 |
1-
Jul- 15 |
2-
Aug -16 |
| US9402812 | Methods for the preparation of liposomes | Indu Javeri | 23-
Sep- 09 |
23-
Sep -10 |
2-
Aug -16 |
| US9394092 | Powdered pouch and method of making same | MONOSOL, LLC | 16-
Apr- 12 |
14-
Mar -13 |
19-
Jul- 16 |
| US9393564 | Bioagent detection systems, devices, and methods | IBIS BIOSCIENCES, INC. | 30-
Mar- 09 |
30-
Mar -10 |
19-
Jul- 16 |
| US9393295 | Nanoparticles for use in pharmaceutical compositions | Novartis AG | 28-
Apr- 08 |
28-
Apr -09 |
19-
Jul- 16 |
| US9393215 | Nanoparticles for use in immunogenic compositions | Novartis AG | 2-
Dec- 05 |
1-
Dec -06 |
19-
Jul- 16 |
| US9388429 | Method for propagating adenoviral vectors encoding inhibitory gene products | GenVec, Inc. | 10-
Nov- 05 |
28-
May -14 |
12-
Jul- 16 |
| US9388234 | Systems and methods for identifying Replikin Scaffolds and uses of said Replikin Scaffolds | Not Available | 6-
Jun- 03 |
19-
Jun -13 |
12-
Jul- 16 |
| US9388198 | Heterocyclic amide derivatives as P2X7 receptor antagonists | ACTELION PHARMACEUTICALS LTD. | 21-
Jan- 14 |
21-
Jan -14 |
12-
Jul- 16 |
| US9388197 | Heterocyclic amide derivatives as P2X7 receptor antagonists | ACTELION PHARMACEUTICALS LTD. | 22-
Jan- 13 |
21-
Jan -14 |
12-
Jul- 16 |
| US9387242 | Chimeric viruses presenting non-native surface proteins and uses thereof | Icahn School of Medicine at Mount Sinai | 2-
Dec- 05 |
1-
Dec -06 |
12-
Jul- 16 |
| US9382590 | Methods and compositions for prostate cancer metastasis | FLORIDA AGRICULTURAL AND MECHANICAL UNIVERSITY (FAMU) | 25-
Mar- 11 |
20-
May -15 |
5-
Jul- 16 |
| US9382545 | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity | COLEY PHARMACEUTICAL GMBH | 27-
Sep- 06 |
4-
Oct -13 |
5-
Jul- 16 |
| US9382288 | Derivatives of steroid benzylamines, having an antiparasitic antibacterial, antimycotic and/or antiviral action | Justus-Liebig-Universitat Giessen | 6-
Oct- 10 |
6-
Oct -11 |
5-
Jul- 16 |
| US9381244 | VISTA modulators for diagnosis and treatment of cancer | KING’S COLLEGE LONDON | 7-
Sep- 12 |
9-
Sep -13 |
5-
Jul- 16 |
| US9381239 | VLPS derived from cells that do not express a viral matrix or core protein | Novavax, Inc. | 25-
May- 07 |
14-
Apr -14 |
5-
Jul- 16 |
| US9381226 | Methods and compositions related to inhibition of viral entry | UNIVERSITY OF UTAH RESEARCH FOUNDATION | 8-
Feb- 07 |
8-
Feb -08 |
5-
Jul- 16 |
| US9381220 | Sceletium extract and uses thereof | H. L. Hall & Sons Limited | 20-
Mar- 09 |
15-
Jul- 14 |
5-
Jul- 16 |
| US9380785 | Antiviral resin member | NBC MESHTEC, INC. | 6-Jul-
11 |
6-
Jul- 12 |
5-
Jul- 16 |
| US9376486 | Human monoclonal antibody with specificity for Dengue virus serotype 1 E protein and uses thereof | DSO National Laboratories | 14-
Dec- 10 |
14-
Dec -11 |
28-
Jun- 16 |
| US9376398 | Carboxylic acid compounds | Astrazeneca Aktiebolag | 18-
May- 12 |
17-
May -13 |
28-
Jun- 16 |
| US9375465 | Conjugates of GM-CSF and IL-7, compositions and methods related thereto | Children’s Healthcare of Atlanta, Inc. | 14-
Nov- 11 |
13-
Nov -12 |
28-
Jun- 16 |
| US9372156 | System for processing contents of a receptacle to detect an optical signal emitted by the contents | GEN-PROBE INCORPORATED | 10-
Mar- 05 |
22-
Feb -11 |
21-
Jun- 16 |
| US9371563 | Nanoreporters and methods of manufacturing and use thereof | NanoString Technologies, Inc. | 23-
Dec- 05 |
11-
Mar -13 |
21-
Jun- 16 |
| US9370582 | Carbohydrate conjugates as delivery agents for oligonucleotides | ALNYLAM PHARMACEUTICALS, INC. | 4-
Dec- 07 |
11-
Jul- 14 |
21-
Jun- 16 |
| US9370581 | Carbohydrate conjugates as delivery agents for oligonucleotides | ALNYLAM PHARMACEUTICALS, INC. | 4-
Dec- 07 |
11-
Jul- 14 |
21-
Jun- 16 |
| US9370570 | Polychlorinated biphenyls and squalene-containing adjuvants | Novartis AG | 28-
Dec- 07 |
10-
Dec -13 |
21-
Jun- 16 |
| US9370531 | Method of providing patient specific immune response in amyloidoses and protein aggregation disorders | New York University | 31-
Aug- 07 |
1-
Sep -08 |
21-
Jun- 16 |
| US9365577 | Pyrimidinone compounds as human neutrophil elastase inhibitors | Chiesi Farmaceutici S.p.A. | 18-
Dec- 12 |
17-
Dec -13 |
14-
Jun- 16 |
| US9365567 | Alkoxy substituted imidazoquinolines | 3M Innovative Properties Company | 3-
Oct- 03 |
30-
Sep -14 |
14-
Jun- 16 |
| US9365523 | Imidazolyl amide compounds and uses related thereto | Children’s Healthcare of Atlanta, Inc. | 31-
Mar- 11 |
28-
Mar -12 |
14-
Jun- 16 |
| US9365506 | Compounds and compositions as TLR2 agonists | NOVARTIS AG | 23-
Mar- 10 |
9-
Jul- 14 |
14-
Jun- 16 |
| US9364511 | Antiviral preparations obtained from a natural cinnamon extract | RAMOT AT TEL-AVIV UNIVERSITY LTD. | 24-
Dec- 03 |
22-
Jun -06 |
14-
Jun- 16 |
| US9359360 | TLR agonists | The Regents of The University of California | 22-
Aug- 05 |
20-
Nov -12 |
7-
Jun- 16 |
| US9358280 | Decreasing potential iatrogenic risks associated with influenza vaccines | Novartis AG | 9-
Sep- 04 |
11-
Jan -13 |
7-
Jun- 16 |
| US9353133 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
7-
Mar -14 |
31-
May -16 |
| US9352048 | Carbohydrate conjugates as delivery agents for oligonucleotides | ALNYLAM PHARMACEUTICALS, INC. | 4-
Dec- 07 |
22-
Jul- 14 |
31-
May -16 |
| US9347055 | Method and kit for preparation of sample for use in nucleic acid amplification | EIKEN KAGAKU KABUSHIKI KAISHA | 5-
Nov- 07 |
5-
Nov -08 |
24-
May -16 |
| US9346866 | Inhibition of tace activity with cyclic peptides | The Regents of the University of California | 2-
Jun- 11 |
19-
Sep -14 |
24-
May -16 |
| US9346794 | Substituted 4-pyridones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 23-
Aug- 12 |
29-
Dec -15 |
24-
May -16 |
| US9346769 | Tetrazolones as inhibitors of fatty acid synthase | Infinity Pharmaceuticals, Inc. | 5-
May- 10 |
23-
Aug -13 |
24-
May -16 |
| US9346753 | Dithiol mucolytic agents | PARION SCIENCES, INC. | 23-
Aug- 13 |
13-
Aug -14 |
24-
May -16 |
| US9345760 | IPNV-ISAV bivalent vaccine using a virus-like particle-based platform and methods of using the same | Advanced Bionutrition Corporation | 9-
Sep- 11 |
7-
Sep -12 |
24-
May -16 |
| US9340507 | Substituted 4-pyridones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 23-
Aug- 12 |
2-
Sep -15 |
17-
May -16 |
| US9339561 | Mutant protease biosensors with enhanced detection characteristics | PROMEGA CORPORATION | 11-
May- 10 |
5-
May -14 |
17-
May -16 |
| US9339525 | Inhibition of biofilm organisms | Novabiotics Limited | 31-
Mar- 09 |
31-
Mar -10 |
17-
May -16 |
| US9334268 | 4-amino-imidazoquinoline compounds | HOFFMAN-LA ROCHE INC. | 22-
Apr- 14 |
22-
Apr -15 |
10-
May -16 |
| US9328110 | Substituted imidazo ring systems and methods | 3M INNOVATIVE PROPERTIES COMPANY | 25-
Nov- 03 |
11-
Mar -14 |
3-
May -16 |
| US9328093 | Selective inhibitors of ubiquitin specific protease 7, the pharmaceutical compositions thereof and their therapeutic applications | HYBRIGENICS SA | 15-
Jan- 10 |
17-
Sep -14 |
3-
May -16 |
| US9326972 | Use of phenylmethimazoles, methimazole derivatives, and tautomeric cyclic thiones for the treatment of autoimmune/inflammatory diseases associated with toll-like receptor overexpression | Ohio University | 16-
Mar- 04 |
9-
Feb -12 |
3-
May -16 |
| US9322827 | B-cell antigen presenting cell assay | University of Pittsburgh – Of the Commonwealth System of Higher Education | 8-
Apr- 10 |
8-
Apr -11 |
26-
Apr- 16 |
| US9321999 | Compositions for increasing polypeptide stability and activity, and related methods | SOLIS BIODYNE OAre | 19-
Nov- 09 |
19-
Nov -10 |
26-
Apr- 16 |
| US9321847 | Activatable toxin complexes comprising a cleavable inhibitory peptide | RAMOT at Tel Aviv University Ltd. | 20-
Sep- 10 |
22-
Aug -11 |
26-
Apr- 16 |
| US9321831 | RSV-specific binding molecules and means for producing them | MedImmune Limited | 1-
Jun- 07 |
13-
Sep -13 |
26-
Apr- 16 |
| US9320784 | Peptides shared among lethal cancers and therapeutic compositions comprising said peptides | Not Available | 7-
Aug- 09 |
19-
Jul- 12 |
26-
Apr- 16 |
| US9320748 | Immunologically useful arginine salts | Novartis AG | 7-
Mar- 12 |
7-
Mar -13 |
26-
Apr- 16 |
| US9315530 | Adsorption of immunopotentiators to insoluble metal salts | Novartis AG | 1-
Sep- 10 |
1-
Sep -11 |
19-
Apr- 16 |
| US9310375 | Luminophore-labeled molecules coupled with particles for microarray-based assays | CapitalBio Corporation | 27-
Oct- 10 |
27-
Oct -10 |
12-
Apr- 16 |
| US9310088 | Device and method for reducing spread of microorganisms and airborne health hazardous matter and/or for protection from microorganisms and airborne health hazardous matter | Technical University of Denmark | 17-
Jul-09 |
14-
Jul- 10 |
12-
Apr- 16 |
| US9309325 | Antibodies and methods of use thereof | The Regents of the University of California | 7-
May- 09 |
4-
May -10 |
12-
Apr- 16 |
| US9303068 | D-amino acid derivative-modified peptidoglycan and methods of use thereof | The Regents of the University of California | 30-
Nov- 12 |
27-
Nov -13 |
5-
Apr- 16 |
| US9303000 | Olefin containing nuclear transport modulators and uses thereof | KARYOPHARM THERAPEUTICS INC. | 17-
Jan- 11 |
16-
Jan -12 |
5-
Apr- 16 |
| US9297010 | Short interfering RNA (siRNA) analogues | Roche Innovation Center Copenhagen A/S | 21-
Mar- 03 |
11-
Feb -14 |
29-
Mar -16 |
| US9295732 | Conjugated TLR7 and/or TLR8 and TLR2 polycationic agonists | INVIVOGEN | 22-
Feb- 13 |
22-
Feb -13 |
29-
Mar -16 |
| US9295708 | Modified release formulations for oprozomib | Onyx Therapeutics, Inc. | 24-
Oct- 12 |
24-
Oct -13 |
29-
Mar -16 |
| US9295646 | Cationic oil-in-water emulsions | Novartis AG | 6-Jul-
10 |
18-
Sep -11 |
29-
Mar -16 |
| US9291628 | Direct clone analysis and selection technology | Dublin City University | 13-
Jul-10 |
13-
Jul- 11 |
22-
Mar -16 |
| US9291597 | Detecting targets using mass tags and mass spectrometry | VENTANA MEDICAL SYSTEMS, INC. | 2-Jul-
10 |
1-
Jul- 11 |
22-
Mar -16 |
| US9290794 | Mutant protease biosensors with enhanced detection characteristics | PROMEGA CORPORATION | 11-
May- 10 |
12-
Nov -12 |
22-
Mar -16 |
| US9290786 | Monoclonal antibody production by EBV transformation of B cells | Institute for Research in Biomedicine | 26-
Feb- 03 |
25-
Apr -13 |
22-
Mar -16 |
| US9290760 | Modified iRNA agents | ALNYLAM PHARMACEUTICALS, INC. | 15-
Sep- 10 |
14-
Sep -11 |
22-
Mar -16 |
| US9290745 | Luciferase biosensor | PROMEGA CORPORATION | 10-
Oct- 03 |
14-
Feb -14 |
22-
Mar -16 |
| US9290545 | Compositions and methods for the treatment of viral infections | Dana-Farber Cancer Institute, Inc. | 23-
Jan- 08 |
23-
Jul- 10 |
22-
Mar -16 |
| US9290459 | Substituted bicyclic dihydropyrimidinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 6-
Feb- 13 |
9-
Nov -15 |
22-
Mar -16 |
| US9290457 | Substituted dihydropyrimidinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 31-
Jul-14 |
27-
Jul- 15 |
22-
Mar -16 |
| US9289487 | II-key/antigenic epitope hybrid peptide vaccines | Antigen Express, Inc. | 14-
Sep- 99 |
11-
Jan -05 |
22-
Mar -16 |
| US9284560 | Application of highly conserved domain sequences from viral genome as template to design therapeutic sliRNAs | Biocross Institute of Molecular Medicine (Nantong) Co., Ltd. | 19-
Sep- 11 |
19-
Sep -11 |
15-
Mar -16 |
| US9278128 | Vaccines and immunotherapeutics comprising IL-15 receptor alpha and/or nucleic acid molecules encoding the same, and methods for using the same | The Trustees of the University of Pennsylvania | 14-
Sep- 09 |
6-
Jan -14 |
8-
Mar -16 |
| US9278126 | Influenza vaccines with reduced amounts of squalene | Seqirus UK Limited | 10-
Feb- 09 |
10-
Feb -10 |
8-
Mar -16 |
| US9272024 | Compositions, comprising improved IL-12 genetic constructs and vaccines, immunotherapeutics and methods of using the same | The Trustees of the University of Pennsylvania | 12-
Dec- 11 |
11-
Dec -12 |
1-
Mar -16 |
| US9271494 | Shelf stable, reduced corrosion, ready to use peroxycarboxylic acid antimicrobial compositions | Ecolab USA, Inc. | 30-
Aug- 07 |
30-
Aug -07 |
1-
Mar -16 |
| US9266844 | Suppression of SARS replication by SARS helicase inhibitors | The Curators of the University of Missouri | 15-
Jun- 12 |
17-
Jun -13 |
23-
Feb -16 |
| US9266843 | Nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 9-
May- 12 |
9-
May -13 |
23-
Feb -16 |
| US9265876 | Systems and methods for pathogen inactivation in blood using UV irradiation while minimizing heat transfer thereto | Hemalux Technologies LLC | 22-
Oct- 14 |
22-
Oct -14 |
23-
Feb -16 |
| US9260398 | Dendrimer like amino amides possessing sodium channel blocker activity for the treatment of dry eye and other mucosal diseases | PARION SCIENCES, INC. | 29-
May- 12 |
9-
Dec -14 |
16-
Feb -16 |
| US9255144 | Anti-IL-18 antibodies and their uses | MedImmune Limited | 20-
Dec- 10 |
20-
Dec -11 |
9-
Feb -16 |
| US9255140 | Adjuvancy and immune potentiating properties of natural products of Onchocerca volvulus | NEW YORK BLOOD CENTER, INC. | 15-
Jun- 04 |
23-
Mar -09 |
9-
Feb -16 |
| US9254315 | Systems and methods for identifying replikin scaffolds and uses of said replikin scaffolds | Not Available | 28-
Apr- 04 |
3-
Feb -10 |
9-
Feb -16 |
| US9254265 | Small liposomes for delivery of immunogen encoding RNA | NOVARTIS AG | 31-
Aug- 10 |
31-
Aug -11 |
9-
Feb -16 |
| US9249427 | Recombinant HCMV and RHCMV vectors and uses thereof | Oregon Health & Science University | 14-
May- 10 |
14-
Nov -12 |
2-
Feb -16 |
| US9249195 | Reovirus vaccines and methods of use therefor | Vanderbilt University | 7-
Apr- 10 |
4-
Apr -11 |
2-
Feb -16 |
| US9248201 | Mutant protease biosensors with enhanced detection characteristics | PROMEGA CORPORATION | 11-
May- 10 |
5-
May -14 |
2-
Feb -16 |
| US9248178 | Different serotypes of vesicular stomatitis virus as expression vectors for immunization regimens | Not Available | 8-
Jun- 09 |
8-
Jun -10 |
2-
Feb -16 |
| US9242980 | Lipidated immune response modifier compound compositions, formulations, and methods | 3M Innovative Properties Company | 17-
Aug- 10 |
16-
Aug -11 |
26-
Jan- 16 |
| US9238809 | Compositions, methods, and kits for isolating and analyzing nucleic acids using an anion exchange material | QIAGEN GAITHERSBURG, INC. | 24-
Sep- 09 |
5-
Aug -10 |
19-
Jan- 16 |
| US9234175 | Creating bioengineered lymph nodes | H. Lee Moffitt Cancer Center and Research Institute, Inc. | 17-
Nov- 09 |
16-
Nov -10 |
12-
Jan- 16 |
| US9233148 | Replikin-based compounds for prevention and treatment of influenza and methods of differentiating infectivity and lethality in influenza | Not Available | 9-
Jan- 09 |
16-
Oct -09 |
12-
Jan- 16 |
| US9227977 | Phosphoinositide 3-kinase inhibitors | Respivert Ltd. | 15-
Mar- 13 |
14-
Mar -14 |
5-
Jan- 16 |
| US9222075 | Animal protein-free media for cultivation of cells | Baxalta GmbH | 29-
Oct- 04 |
2-
May -14 |
29-
Dec -15 |
| US9221832 | Heterocyclic amide derivatives as P2X7 receptor antagonists | ACTELION PHARMACEUTICALS LTD. | 22-
Jul-11 |
20-
Jul- 12 |
29-
Dec -15 |
| US9221807 | Substituted pyridones and pyrazinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 21-
Feb- 14 |
12-
Feb -15 |
29-
Dec -15 |
| US9220768 | Decreasing potential iatrogenic risks associated with influenza vaccines | Novartis AG | 9-
Sep- 04 |
14-
Oct -11 |
29-
Dec -15 |
| US9217745 | Arrayed detector system for measurement of influenza immune response | University of Rochester | 2-
May- 08 |
11-
Jul- 13 |
22-
Dec -15 |
| US9217157 | Recombinant influenza viruses and uses thereof | Icahn School of Medicine at Mount Sinai | 27-
Jul-09 |
27-
Jul- 10 |
22-
Dec -15 |
| US9216192 | Toll-like receptor agonist formulations and their use | VentiRx Pharmaceuticals, Inc. | 1-
Aug- 08 |
16-
Jul- 12 |
22-
Dec -15 |
| US9213027 | Lipoparticles comprising proteins, methods of making, and using the same | Integral Molecular, Inc. | 30-
Jul-03 |
1-
Nov -13 |
15-
Dec -15 |
| US9212399 | Biological specimen collection and transport system and method of use | Longhorn Vaccines and Diagnostics, LLC | 1-
Oct- 07 |
7-
Jan -14 |
15-
Dec -15 |
| US9212205 | Nucleic acid binding compounds and methods of use | University of Rochester | 26-
Jul-07 |
28-
Jul- 08 |
15-
Dec -15 |
| US9206396 | Methods and devices for quantitative viral assays | Wisconsin Alumni Research Foundation | 16-
Nov- 05 |
16-
Nov -06 |
8-
Dec -15 |
| US9206158 | Hydrazide containing nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 29-
Jul-11 |
10-
Jun -15 |
8-
Dec -15 |
| US9200329 | Rapid epidemiologic typing of bacteria | BioFire Diagnostics, LLC | 19-
May- 08 |
18-
May -09 |
1-
Dec -15 |
| US9200287 | Phosphate-modified oligonucleotide analogs with enhanced immunostimulatory activity | AdiuTide Pharmaceuticals GmbH | 18-
May- 07 |
15-
May -08 |
1-
Dec -15 |
| US9200280 | Methods and compositions for the treatment of cancer or other diseases | CITY OF HOPE | 26-
Jan- 07 |
9-
Jun -14 |
1-
Dec -15 |
| US9200279 | Methods and compositions for the treatment of cancer or other diseases | CITY OF HOPE | 26-
Jan- 07 |
14-
Jan -14 |
1-
Dec -15 |
| US9200074 | Antibodies to IL-1 R1 and methods of making them | MEDIMMUNE LIMITED | 7-
Nov- 08 |
16-
Apr -14 |
1-
Dec -15 |
| US9199981 | Compounds and compositions as C-kit kinase inhibitors | NOVARTIS AG | 1-
Sep- 11 |
27-
Aug -12 |
1-
Dec -15 |
| US9199897 | Methods for preparing squalene | NOVARTIS AG | 12-
May- 10 |
12-
May -11 |
1-
Dec -15 |
| US9198927 | Targeting opposite strand replication intermediates of singlestranded viruses by RNAI | ALNYLAM PHARMACEUTICALS, INC. | 24-
Sep- 04 |
1-
Mar -10 |
1-
Dec -15 |
| US9193780 | Amino acid sequences directed against envelope proteins of a virus and polypeptides comprising the same for the treatment of viral diseases | Ablynx N.V. | 5-
Jun- 08 |
5-
Jun -09 |
24-
Nov -15 |
| US9192661 | Delivery of self-replicating RNA using biodegradable polymer particles | Novartis AG | 6-Jul-
10 |
7-
Jun -11 |
24-
Nov -15 |
| US9187748 | Compositions and methods for silencing ebola virus gene expression | Not Available | 20-
Jul-09 |
28-
Mar -14 |
17-
Nov -15 |
| US9187426 | Organic compounds | Novartis AG | 27-
Jun- 08 |
25-
Jun -09 |
17-
Nov -15 |
| US9186419 | Directed evolution and in vitro panning of virus vectors | The University of North Carolina at Chapel Hill | 30-
Apr- 08 |
17-
Jan -14 |
17-
Nov -15 |
| US9186399 | Immune stimulatory oligonucleotide analogs containing modified sugar moieties | AdiutTide Pharmaceuticals GmbH | 9-
Oct- 07 |
29-
Sep -08 |
17-
Nov -15 |
| US9181303 | Treatment of bacterial infections with cyclic antimicrobial peptides | NovaBiotics Limited | 22-
Dec- 05 |
20-
Jun -14 |
10-
Nov -15 |
| US9181290 | Inhibition of biofilm formation by 1,2,3,4,6-penta-O-galloyl-D- glucopyranose | CHANG GUNG UNIVERSITY | 17-
Jun- 11 |
19-
Sep -11 |
10-
Nov -15 |
| US9175047 | Peptidomimetic macrocycles | Aileron Therapeutics, Inc. | 14-
Jan- 09 |
14-
Jan -10 |
3-
Nov -15 |
| US9174925 | Phorbol type diterpene compound, pharmaceutical composition for treatment or prevention of viral infectious diseases including same | KOREA RESEARCH INSTITUTE OF BIOSCIENCE AND BIOTECHNOLOGY | 26-
Oct- 11 |
28-
Sep -12 |
3-
Nov -15 |
| US9169318 | Neutralizing molecules to viral antigens | Sea Lane Biotechnologies, Inc. | 28-
Mar- 08 |
18-
Jul- 11 |
27-
Oct- 15 |
| US9168318 | Oxidative reductive potential water solution and methods of using the same | Oculus Innovative Sciences, Inc. | 30-
Dec- 03 |
11-
Aug -04 |
27-
Oct- 15 |
| US9168299 | Methods for treating juvenile arthritis with ant-bile salt-stimulated lipase (BSSL) antibodies | LIPUM AB | 8-
Apr- 09 |
30-
Oct -13 |
27-
Oct- 15 |
| US9168269 | Inhibitors of long and very long chain fatty acid metabolism as broad spectrum anti-virals | THE TRUSTEES OF PRINCETON UNIVERSITY | 18-
Feb- 10 |
18-
Feb -11 |
27-
Oct- 15 |
| US9163222 | Mutations in OAS1 genes | Kineta Two, LLC | 4-
May- 05 |
14-
Nov -12 |
20-
Oct- 15 |
| US9163065 | Depsipeptide and uses thereof | NovoBiotic Pharmaceuticals, LLC | 3-
Dec- 12 |
3-
Dec -13 |
20-
Oct- 15 |
| US9161976 | Immunotherapy comprising TLR9 ligand and CD40 ligand | Trustees of Dartmouth College | 30-
Dec- 02 |
22-
Oct -12 |
20-
Oct- 15 |
| US9156811 | N-myristoyl transferase inhibitors | Univeristy of Dundee | 2-
Sep- 08 |
29-
Aug -09 |
13-
Oct- 15 |
| US9155309 | Virus inactivating sheet | NBC MESHTEC, INC. | 2-
Oct- 09 |
4-
Oct -10 |
13-
Oct- 15 |
| US9149473 | Targeted whole genome amplification method for identification of pathogens | IBIS BIOSCIENCES, INC. | 14-
Sep- 06 |
14-
Sep -07 |
6-
Oct- 15 |
| US9149445 | Inhibition of glycerol-3-phosphate acyltransferase (GPAT) and associated enzymes for treatment of viral infections | THE TRUSTEES OF PRINCETON UNIVERSITY | 27-
Jul-09 |
27-
Jul- 10 |
6-
Oct- 15 |
| US9145588 | Generation of binding molecules | MERUS BIOPHARMACEUTICALS B.V. | 26-
Sep- 11 |
26-
Sep -12 |
29-
Sep -15 |
| US9145585 | Method for using permuted nucleic acid probes | Ventana Medical Systems, Inc. | 1-
Sep- 06 |
5-
Aug -14 |
29-
Sep -15 |
| US9145410 | Pyrazolopyridines and analogs thereof | 3M Innovative Properties Company | 3-
Oct- 03 |
26-
Jan -12 |
29-
Sep -15 |
| US9144575 | Anti-viral azide containing compounds | LIFE TECHNOLOGIES CORPORATION | 28-
Jul-10 |
28-
Jul- 11 |
29-
Sep -15 |
| US9139833 | Modified small interfering RNA molecules and methods of use | Arrowhead Research Corporation | 26-
Jul-02 |
12-
Mar -13 |
22-
Sep -15 |
| US9139647 | Diagnosis and treatment of cancer using anti-TM4SF20 antibody | FORERUNNER PHARMA RESEARCH CO., LTD. | 25-
Dec- 08 |
25-
Dec -09 |
22-
Sep -15 |
| US9139620 | Feline morbillivirus and uses thereof | THE GOVERNMENT OF THE HONG KONG SPECIAL ADMINISTRATIVE REGION OF THE PEOPLE’S REPUBLIC OF CHINA | 20-
Jan- 12 |
22-
Jan -13 |
22-
Sep -15 |
| US9138472 | CD40L vaccines, compositions, and methods related thereto | EMORY UNIVERSITY | 28-
Sep- 10 |
28-
Sep -11 |
22-
Sep -15 |
| US9134247 | Method and apparatus for two-step surface-enhanced raman spectroscopy | REAL-TIME ANALYZERS, INC. | 16-
Dec- 11 |
16-
Dec -11 |
15-
Sep -15 |
| US9133248 | Methods of propagating monkey adenoviral vectors | GenVec, Inc. | 9-
Nov- 09 |
9-
Nov -10 |
15-
Sep -15 |
| US9132423 | Sample-to-answer microfluidic cartridge | Micronics, Inc. | 29-
Jan- 10 |
28-
Jan -11 |
15-
Sep -15 |
| US9132175 | Bacillus based delivery system and methods of use | The Curators of the University of Missouri | 18-
Apr- 11 |
18-
Apr -11 |
15-
Sep -15 |
| US9128101 | Biomarkers for theranostics | Caris Life Sciences Switzerland Holdings GmbH | 1-
Mar- 10 |
1-
Mar -11 |
8-
Sep -15 |
| US9127256 | Method for production of reprogrammed cell using chromosomally unintegrated virus vector | DNAVEC CORPORATION | 16-
Jul-08 |
16-
Jul- 09 |
8-
Sep -15 |
| US9127251 | Means and methods for influencing the stability of antibody producing cells | ACADEMISCH MEDISCH CENTRUM BIJ DE UNIVERSITEIT VAN AMSTERDAM | 9-
Dec- 05 |
8-
Dec -06 |
8-
Sep -15 |
| US9127028 | Substrates for chromogenic detection and methods of use in detection assays and kits | Ventana Medical Systems, Inc. | 16-
Aug- 10 |
12-
Aug -11 |
8-
Sep -15 |
| US9125952 | Immunostimulatory compositions comprising liposome-encapsulated oligonucleotides and epitopes | Industry Academic Cooperation Foundation, Hallym University | 17-
Jul-09 |
13-
Aug -14 |
8-
Sep -15 |
| US9115093 | Substituted bicyclic dihydropyrimidinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 4-
Mar- 13 |
20-
Feb -14 |
25-
Aug -15 |
| US9115065 | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with Arenaviruses | Kineta, Inc. | 6-
Dec- 04 |
26-
Feb -13 |
25-
Aug -15 |
| US9109199 | Methods to produce bunyavirus replicon particles | STICHTING DIENST LANDBOUWKUNDIG ONDERZOEK | 20-
Sep- 10 |
20-
Sep -11 |
18-
Aug -15 |
| US9107970 | Method and a filter for capturing airborne agents | Not Available | 15-
Jul-08 |
13-
Jul- 09 |
18-
Aug -15 |
| US9107958 | Hydrazino 1H-imidazoquinolin-4-amines and conjugates made therefrom | 3M Innovative Properties Company | 3-
Jun- 11 |
1-
Jun -12 |
18-
Aug -15 |
| US9107906 | Compositions and methods for the treatment of immunodeficiency | ADMA BIOLOGICS, INC. | 28-
Oct- 14 |
8-
Jan -15 |
18-
Aug -15 |
| US9107904 | Immunostimulatory compositions and methods of use thereof | Massachusetts Institute of Technology | 5-
Apr- 12 |
15-
Mar -13 |
18-
Aug -15 |
| US9102938 | 2â€2 and 5â€2 modified monomers and oligonucleotides | ALNYLAM PHARMACEUTICALS, INC. | 31-
Mar- 11 |
31-
Mar -11 |
11-
Aug -15 |
| US9102911 | High density self-contained biological analysis | BioFire Diagnostics, LLC | 15-
May- 09 |
28-
Jan -13 |
11-
Aug -15 |
| US9102741 | GAS57 mutant antigens and GAS57 antibodies | Novartis AG | 12-
Sep- 07 |
13-
Oct -14 |
11-
Aug -15 |
| US9102740 | Cna-B domain antigens in vaccines against gram positive bacteria | NOVARTIS AG | 12-
Jan- 09 |
7-
Jun -13 |
11-
Aug -15 |
| US9102633 | Arylalkyl- and aryloxyalkyl-substituted epithelial sodium channel blocking compounds | Parion Sciences, Inc. | 13-
Dec- 13 |
13-
Dec -13 |
11-
Aug -15 |
| US9102624 | Substituted 4-pyridones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 23-
Aug- 12 |
20-
Aug -13 |
11-
Aug -15 |
| US9101597 | Immunoprotective primary mesenchymal stem cells and methods | Autoimmune Technologies, LLC | 14-
Mar- 13 |
14-
Mar -13 |
11-
Aug -15 |
| US9101582 | Use of a pneumococcal P4 peptide for enhancing opsonophagocytosis in response to a pathogen | The United States of America as represented by the Secretary of the Department of Health and Human Services, Centers for Disease Control and Prevention | 31-
Jul-08 |
27-
Mar -13 |
11-
Aug -15 |
| US9096585 | Antiviral compounds and uses thereof | Icahn School of Medicine at Mount Sinai | 28-
May- 10 |
31-
May -11 |
4-
Aug -15 |
| US9096543 | Nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 9-
May- 12 |
9-
May -13 |
4-
Aug -15 |
| US9090897 | Production of IFN-lambda by conventional dendritic cells | Bavarian Nordic A/S | 18-
Dec- 09 |
17-
Dec -10 |
28-
Jul- 15 |
| US9085641 | Peptides regulating the surface expression of the T cell receptor | Max-Delbruck-Centrum Fur Molekulare Medizin | 23-
Jun- 06 |
23-
Jun -07 |
21-
Jul- 15 |
| US9084808 | Modified small interfering RNA molecules and methods of use | Arrowhead Research Corporation | 1-
Oct- 04 |
9-
May -14 |
21-
Jul- 15 |
| US9084758 | Antiviral compositions comprising ethanol extract of Tetracera scandens and use thereof | The Catholic University of Korea Industry-Academic Cooperation Foundation | 24-
Jul-12 |
25-
Aug -14 |
21-
Jul- 15 |
| US9080209 | Non-mass determined base compositions for nucleic acid detection | IBIS BIOSCIENCES, INC. | 6-
Aug- 09 |
6-
Aug -10 |
14-
Jul- 15 |
| US9080204 | Compositions and methods for rapid, real-time detection of influenza a virus (H1N1) Swine 2009 | Longhorn Vaccines and Diagnostics, LLC | 12-
Sep- 06 |
30-
Dec -11 |
14-
Jul- 15 |
| US9079965 | Bispecific antibody | Wuhan YZY Biopharma Co., LTD. | 21-
Nov- 12 |
13-
Mar -14 |
14-
Jul- 15 |
| US9079943 | TC-83-derived alphavirus vectors, particles and methods | ALPHAVAX, INC. | 18-
May- 04 |
28-
Mar -14 |
14-
Jul- 15 |
| US9079865 | Hydrazide containing nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 29-
Jul-11 |
29-
Jul- 12 |
14-
Jul- 15 |
| US9078885 | Respiratory disease treatment | Pulmagen Therapeutics (Inflammation) Limited | 7-
Aug- 08 |
7-
Apr -14 |
14-
Jul- 15 |
| US9078868 | Therapeutic agent for accelerating recovery of animal under medical treatment | DAIICHI SANKYO COMPANY, LIMITED | 15-
Jan- 10 |
14-
Jan -11 |
14-
Jul- 15 |
| US9073869 | Method of using substituted 2-Aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C | Boehringer Ingelheim International GmbH | 14-
Mar- 13 |
11-
Sep -14 |
7-
Jul- 15 |
| US9072738 | Chemically and metabolically stable dipeptide possessing potent sodium channel blocker activity | PARION SCIENCES, INC. | 27-
Jun- 11 |
27-
Jun -12 |
7-
Jul- 15 |
| US9072726 | Methods of treating or preventing inflammation and hypersensitivity with oxidative reductive potential water solution | Oculus Innovative Sciences, Inc. | 20-
Jan- 06 |
21-
Dec -09 |
7-
Jul- 15 |
| US9072702 | Reverse genetics using non-endogenous pol I promoters | Novartis AG | 21-
May- 09 |
21-
May -10 |
7-
Jul- 15 |
| US9067873 | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses | Kineta Four, LLC | 6-
Dec- 04 |
19-
Dec -13 |
30-
Jun- 15 |
| US9066964 | Use of tylvalosin as antiviral agent | Cambridge University Technical Services | 13-
Jul-06 |
13-
Jul- 07 |
30-
Jun- 15 |
| US9063150 | Method for detection of antigen-specific antibodies in biological samples | The United States of America as represented by the Secretary of the Department of Health and Human Services, Centers for Disease Control Prevention | 2-
Sep- 08 |
25-
Aug -09 |
23-
Jun- 15 |
| US9061001 | Combination adjuvant formulation | Dalhousie University | 16-
Oct- 08 |
15-
Oct -09 |
23-
Jun- 15 |
| US9056900 | Compositions and methods for coronavirus inhibition | Autoimmune Technologies, LLC. | 4-
Nov- 03 |
8-
Aug -13 |
16-
Jun- 15 |
| US9056898 | Attenuated RNA virus and applications thereof | Washington University | 20-
Sep- 07 |
22-
Sep -08 |
16-
Jun- 15 |
| US9056071 | Compounds and methods for preventing or treating a viral infection | CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (C.N.R.S.) | 2-
Nov- 07 |
19-
Jun -13 |
16-
Jun- 15 |
| US9051619 | Methods and compositions for prostate cancer metastasis | FLORIDA AGRICULTURAL AND MECHANICAL UNIVERSITY (FAMU) | 25-
Mar- 11 |
23-
Mar -12 |
9-
Jun- 15 |
| US9051564 | Compositions for and methods of identifying antigens | President and Fellows of Harvard College | 21-
Feb- 06 |
21-
Feb -07 |
9-
Jun- 15 |
| US9051353 | Crystalline tripeptide epoxy ketone protease inhibitors | Onyx Therapeutics, Inc. | 20-
Mar- 09 |
24-
Sep -13 |
9-
Jun- 15 |
| US9050376 | Conjugates of synthetic TLR agonists and uses therefor | The Regents of the University of California | 7-
Feb- 07 |
19-
Jun -14 |
9-
Jun- 15 |
| US9046523 | Rapid bioluminescence detection system | THE SECRETARY OF STATE FOR HEALTH | 7-
Jan- 09 |
2-
Aug -13 |
2-
Jun- 15 |
| US9045855 | Anti-viral member | NBC Meshtec, Inc. | 26-
Dec- 08 |
28-
Dec -09 |
2-
Jun- 15 |
| US9045727 | Virus-like particles, methods of preparation, and immunogenic compositions | EMORY UNIVERSITY | 17-
May- 02 |
4-
Apr -06 |
2-
Jun- 15 |
| US9045472 | Imidazoquinoline compounds | ASTRAZENECA AB | 16-
Dec- 10 |
16-
Dec -11 |
2-
Jun- 15 |
| US9045470 | Compounds and compositions as TLR activity modulators | IRM LLC | 2-
Sep- 09 |
1-
Sep -10 |
2-
Jun- 15 |
| US9044420 | Immunogenic compositions and methods of using the compositions for inducing humoral and cellular immune responses | IMMUNE DESIGN CORP. | 8-
Apr- 11 |
6-
Apr -12 |
2-
Jun- 15 |
| US9040310 | Antibody-nanoparticle conjugates and methods for making and using such conjugates | Ventana Medical Systems, Inc. | 27-
Apr- 10 |
27-
Apr -11 |
26-
May -15 |
| US9034646 | Virally-inactivated growth factors-containing platelet lysate depleted of PDGF and VEGF and preparation method thereof | ZHENG YANG BIOMEDICAL TECHNOLOGY CO., LTD. | 25-
May- 10 |
26-
Nov -12 |
19-
May -15 |
| US9034313 | Nucleic acid molecules encoding rantes, and compositions comprising and methods of using the same | Inovio Pharmaceuticals, Inc. | 8-
Feb- 10 |
8-
Feb -11 |
19-
May -15 |
| US9029413 | T reatment of viral infections by modulation of host cell metabolic pathways | The Trustees of Princeton University | 1-
Jun- 07 |
3-
Apr -12 |
12-
May -15 |
| US9029382 | 3,5-diamino-6-chloro-N-(N-(4-phenylbutyl)carbamimidoyl) pyrazine-2-carboxamide compounds | Parion Sciences, Inc. | 17-
Dec- 12 |
13-
Dec -13 |
12-
May -15 |
| US9029315 | Soluble PD-1 variants, fusion constructs, and uses thereof | The University of Hong Kong | 11-
Nov- 10 |
11-
Nov -11 |
12-
May -15 |
| US9028841 | Synergistic bacterial compositions and methods of production and use thereof | Seres Health, Inc. | 23-
Nov- 12 |
20-
Mar -14 |
12-
May -15 |
| US9028837 | Methods and compositions for poxvirus A35R protein | East Carolina University | 7-
Jun- 12 |
20-
Dec -12 |
12-
May -15 |
| US9028823 | Methods of inducing or enhancing an immune response in a subject by administering agonistic GITR binding antibodies | GITR, Inc. | 25-
Mar- 05 |
1-
Mar -13 |
12-
May -15 |
| US9024001 | Alphavirus replicon packaging constructs | Novartis Vaccines and Diagnostics, Inc. | 25-
May- 04 |
20-
May -05 |
5-
May -15 |
| US9023855 | Compounds | Chiesi Farmaceutici S.p.A. | 14-
Sep- 11 |
11-
Feb -14 |
5-
May -15 |
| US9023839 | Compounds and compositions as c-kit kinase inhibitors | IRM LLC | 1-
Sep- 11 |
22-
Apr -14 |
5-
May -15 |
| US9017699 | Adjuvancy and immune potentiating properties of natural products of Onchocerca volvulus | New York Blood Center, Inc. | 15-
Jun- 04 |
18-
Feb -10 |
28-
Apr- 15 |
| US9017696 | Adenovirus vectors | Isis Innovation Limited | 10-
Apr- 07 |
10-
Apr -08 |
28-
Apr- 15 |
| US9012622 | Compositions and methods using siRNA molecules and siRNA cocktails for the treatment of breast cancer | Not Available | 31-
Dec- 08 |
31-
Dec -09 |
21-
Apr- 15 |
| US9011767 | Transportable vacuum assisted decontamination unit and decontamination process | STERIS Inc. | 1-
Apr- 13 |
31-
Mar -14 |
21-
Apr- 15 |
| US9006264 | Substituted imidazoquinolines, imidazopyridines, and i midazonaphthyridines | 3M Innovative Properties Company | 18-
Jun- 04 |
9-
Sep -13 |
14-
Apr- 15 |
| US9006194 | Compositions and methods for diminishing viral infection and inflammation associated with viral infection | Drexel University | 19-
Dec- 08 |
17-
Dec -09 |
14-
Apr- 15 |
| US9005974 | Means and methods for influencing the stability of cells | Academish Medisch Centrum Bij de Universiteit van Amsterdam | 9-
Dec- 05 |
9-
Dec -05 |
14-
Apr- 15 |
| US9005665 | Compositions and methods for treating and preventing porcine reproductive and respiratory syndrome | Ohio State Innovation Foundation | 24-
Apr- 12 |
24-
Apr -13 |
14-
Apr- 15 |
| US9005599 | Genetically modified human umbilical cord perivascular cells for prophylaxis against or treatment of biological or chemical agents | Tissue Regeneration Therapeutics Inc. | 21-
Apr- 08 |
20-
Apr -09 |
14-
Apr- 15 |
| US8999996 | Hydrazide containing nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 29-
Jul-11 |
19-
Mar -14 |
7-
Apr- 15 |
| US8999975 | Substituted N- [1-cyano-2- (phenyl) ethyl] -2-azabicyclo [2.2.1] heptane-3-carboxamide inhibitors of cathepsin C | Boehringer Ingelheim International GmbH | 19-
Sep- 11 |
14-
Sep -12 |
7-
Apr- 15 |
| US8999678 | Method of increasing the function of an AAV vector | The Trustees of the University of Pennsylvania | 7-
Apr- 05 |
7-
Apr -06 |
7-
Apr- 15 |
| US8999349 | HMGBl-derived peptides enhance immune response to antigens | The Regents of the University of California | 27-
Jul-10 |
27-
Jul- 11 |
7-
Apr- 15 |
| US8999316 | Antiviral compounds | Long Island University | 30-
May- 07 |
30-
May -08 |
7-
Apr- 15 |
| US8993717 | Gadd45beta targeting agents | Imperial Innovations Limited | 22-
Oct- 09 |
22-
Oct -10 |
31-
Mar -15 |
| US8993581 | Methods for treating viral disorders | Trustees of Boston University | 24-
Sep- 09 |
11-
Jun -13 |
31-
Mar -15 |
| US8993295 | Methods, compositions, and kits for the selective activation of protoxins through combinatorial targeting | The General Hospital Corporation | 20-
Jul-06 |
20-
Jul- 07 |
31-
Mar -15 |
| US8992939 | Highly efficient influenza matrix (M1) proteins | Novavax, Inc. | 11-
Jul-03 |
24-
Oct -11 |
31-
Mar -15 |
| US8987249 | Substituted 2-Aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl- cyano-methyl)-amides inhibitors of Cathepsin C | Boehringer Ingelheim International GmbH | 14-
Mar- 13 |
12-
Mar -14 |
24-
Mar -15 |
| US8987191 | Bioactive peptides and methods of using same | Compugen Ltd. | 12-
Jul-07 |
21-
Jun -13 |
24-
Mar -15 |
| US8986933 | Selective detection of human rhinovirus | The United States of America as represented by the Secretary of the Department of Health and Human Services, Centers for Disease Control | 5-
Dec- 08 |
5-
Dec -08 |
24-
Mar -15 |
| US8986926 | Compositions comprising oriented, immobilized macromolecules and methods for their preparation | NanoString Technologies, Inc. | 23-
Dec- 05 |
22-
Dec -06 |
24-
Mar -15 |
| US8986702 | Antibodies and processes for preparing the same | Taiga Biotechnologies, Inc. | 16-
May- 08 |
18-
May -09 |
24-
Mar -15 |
| US8980898 | Dendrimer like amino amides possessing sodium channel blocker activity for the treatment of dry eye and other mucosal diseases | Parion Sciences, Inc. | 29-
May- 12 |
29-
May -13 |
17-
Mar -15 |
| US8980338 | Sceletium extract and uses thereof | H.L. Hall & Sons Limited | 20-
Mar- 09 |
16-
Mar -10 |
17-
Mar -15 |
| US8980281 | High-yield transgenic mammalian expression system for generating virus-like particles | Academia Sinica | 5-
Sep- 06 |
12-
Feb -10 |
17-
Mar -15 |
| US8975389 | Nucleic acid chemical modifications | Alnylam Pharmaceuticals, Inc. | 2-
Mar- 09 |
2-
Mar -10 |
10-
Mar -15 |
| US8969362 | 9-substituted 8-oxoadenine compound | AstraZeneca Aktiebolag | 26-
Mar- 04 |
21-
Oct -13 |
3-
Mar -15 |
| US8969350 | Pharmaceutical product comprising a p38 kinase inhibitor and a second active ingredient | Astrazeneca AB | 18-
Dec- 08 |
17-
Dec -09 |
3-
Mar -15 |
| US8962580 | Chemical modifications of monomers and oligonucleotides with cycloaddition | Alnylam Pharmaceuticals, Inc. | 23-
Sep- 08 |
23-
Sep -09 |
24-
Feb -15 |
| US8962332 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
13-
Sep -13 |
24-
Feb -15 |
| US8962330 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
31-
Oct -07 |
24-
Feb -15 |
| US8961983 | Mucosal vaccine using cationic nanogel | National University Corporation Tokyo Medical and Dental University | 31-
Oct- 08 |
5-
Sep -14 |
24-
Feb -15 |
| US8961477 | Delivery of immune response modifier compounds | 3M Innovative Properties Company | 25-
Aug- 03 |
25-
Aug -04 |
24-
Feb -15 |
| US8956863 | Agents from cells | The Brigham and Women’s Hospital, Inc. | 15-
Oct- 09 |
15-
Oct -10 |
17-
Feb -15 |
| US8956616 | Constructs binding to phosphatidylserine and their use in disease treatment | Board of Regents, The University of Texas System | 24-
Jan- 05 |
24-
Jan -06 |
17-
Feb -15 |
| US8951768 | Mutations in OAS1 genes | Kineta Two, LLC | 4-
May- 05 |
11-
Jul- 11 |
10-
Feb -15 |
| US8951528 | Immune response modifier conjugates | 3M Innovative Properties Company | 22-
Feb- 06 |
21-
Feb -07 |
10-
Feb -15 |
| US8945943 | Personal glucose meters for detection and quantification of a broad range of analytes | The Board of Trustees of the University of Illinois | 26-
May- 10 |
26-
May -11 |
3-
Feb -15 |
| US8945904 | Influenza virus reassortment | Novartis AG | 21-
May- 10 |
20-
May -11 |
3-
Feb -15 |
| US8945610 | Condensation products based on bicyclic or polycyclic aromatics or heteroaromatics | BASF SE | 14-
Nov- 07 |
11-
Nov -08 |
3-
Feb -15 |
| US8940864 | Stabilized therapeutic small helical antiviral peptides | New York Blood Center, Inc. | 5-
Oct- 06 |
2-
Oct -07 |
27-
Jan- 15 |
| US8940501 | Methods for ligation and uses thereof | Whitehead Institute for Biomedical Research | 30-
Jan- 09 |
1-
Feb -10 |
27-
Jan- 15 |
| US8937154 | Stabilized therapeutic small helical antiviral peptides | New York Blood Center, Inc. | 5-
Oct- 06 |
2-
Feb -12 |
20-
Jan- 15 |
| US8933210 | Label-free functional nucleic acid sensors for detecting target agents | The Board of Trustees of the University of Illinois | 6-
Oct- 10 |
6-
Oct -11 |
13-
Jan- 15 |
| US8933019 | Antiviral cell-penetrating peptides | New York Blood Center, Inc. | 6-
May- 08 |
31-
Oct -12 |
13-
Jan- 15 |
| US8916552 | Pharmaceutical combinations | Astex Therapeutics Limited | 12-
Oct- 06 |
12-
Oct -07 |
23-
Dec -14 |
| US8916340 | Method for identifying and validating dominant T helper cell epitopes using an HLA-DM-assisted class II binding assay | The John Hopkins University | 6-
Jan- 06 |
8-
Jan -07 |
23-
Dec -14 |
| US8906872 | Antisense antiviral compound and method for treating ssRNA viral infection | Sarepta Therapeutics, Inc. | 16-
Sep- 04 |
22-
Dec -11 |
9-
Dec -14 |
| US8906863 | Proteolysis-resistant capsid of chimeric hepatitis E virus as an oral delivery vector | The Regents of the University of California | 27-
Feb- 09 |
1-
Sep -11 |
9-
Dec -14 |
| US8906862 | Multiple antigen delivery system using hepatitis E virus-like particle | National Institute of Infectious Disease | 27-
Feb- 09 |
29-
Aug -11 |
9-
Dec -14 |
| US8901071 | Compounds and their use | Novabiotics Limited | 31-
Mar- 10 |
30-
Mar -11 |
2-
Dec -14 |
| US8900585 | Influenza hemagglutinin-specific monoclonal antibodies for preventing and treating influenza virus infection | New York Blood Center, Inc. | 20-
Oct- 10 |
20-
Oct -11 |
2-
Dec -14 |
| US8895629 | Circulation of components during homogenization of emulsions | Novartis AG | 3-
Dec- 09 |
3-
Dec -10 |
25-
Nov -14 |
| US8895577 | Compounds and compositions as TLR activity modulators | Not Available | 3-
Mar- 08 |
22-
Apr -13 |
25-
Nov -14 |
| US8895570 | Purine derivatives | AstraZeneca AB | 17-
Dec- 10 |
14-
Dec -11 |
25-
Nov -14 |
| US8895534 | Boron containing small molecules | Anacor Pharmaceuticals, Inc. | 20-
Jun- 07 |
30-
Jul- 10 |
25-
Nov -14 |
| US8895295 | High density self-contained biological analysis | Biofire Diagnostics, LLC | 15-
Nov- 06 |
14-
Nov -07 |
25-
Nov -14 |
| US8889708 | Substituted bicyclic 1-carboxylic-acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C | Boehringer Ingelheim International GmbH | 14-
Mar- 13 |
12-
Mar -14 |
18-
Nov -14 |
| US8889692 | Pyrazinone derivatives, pharmaceutically acceptance salts thereof and their uses | AstraZeneca AB | 27-
Jun- 07 |
14-
Sep -12 |
18-
Nov -14 |
| US8889656 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
30-
Apr -13 |
18-
Nov -14 |
| US8889398 | Composition for inactivating an enveloped virus | Viroblock SA | 19-
May- 06 |
18-
May -07 |
18-
Nov -14 |
| US8889181 | Immunostimulatory compositions comprising liposome-encapsulated oligonucleotides and epitopes | Industry Academic Cooperation Foundation, Hallym University | 17-
Jul-09 |
16-
Jun -10 |
18-
Nov -14 |
| US8889118 | Anticancer agent containing dendritic cell having RNA virus transferred thereinto | DNA VEC Research Inc. | 24-
Jun- 04 |
28-
Apr -05 |
18-
Nov -14 |
| US8889117 | Modular nanoparticles for adaptable vaccines | Yale University | 15-
Feb- 07 |
15-
Feb -08 |
18-
Nov -14 |
| US8884020 | Indole compounds | Ironwood Pharmaceuticals, Inc. | 7-
Aug- 06 |
7-
Aug -07 |
11-
Nov -14 |
| US8883790 | Pharmaceutical combinations | Astex Therapeutics Limited | 12-
Oct- 06 |
12-
Oct -07 |
11-
Nov -14 |
| US8883500 | Method of preparing adenosine-resistant anti-tumor T lymphocytes for adoptive immunotherapy | Northeastern University | 5-
Dec- 08 |
7-
Dec -09 |
11-
Nov -14 |
| US8883481 | Reverse genetics methods for virus rescue | Novartis AG | 20-
Oct- 09 |
20-
Oct -10 |
11-
Nov -14 |
| US8883477 | Oligoadenylate synthetase (OAS) | Kineta Two, LLC | 23-
Nov- 05 |
14-
Jun -13 |
11-
Nov -14 |
| US8882484 | Methods and compositions for production of recombinant protein in HBX-expressing mammalian cells | Bayer Healthcare LLC | 28-
May- 08 |
27-
May -09 |
11-
Nov -14 |
| US8881040 | System and method for detecting, collecting, analyzing, and communicating event-related information | Georgetown University | 28-
Aug- 08 |
2-
Dec -09 |
4-
Nov -14 |
| US8877775 | Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl- cyano-methyl)-amides inhibitors of cathepsin C | Boehringer Ingelheim International GmbH | 14-
Mar- 13 |
12-
Mar -14 |
4-
Nov -14 |
| US8877187 | Therapeutic antibodies for treatment and prophylaxis of transmittable viral diseases | Avianax, LLC | 25-
Jul-05 |
23-
Nov -10 |
4-
Nov -14 |
| US8877060 | Methods for removing pathogens from a platelet preparation | Biovec Transfusion, LLC | 23-
Nov- 10 |
31-
Oct -11 |
4-
Nov -14 |
| US8871816 | Methods for producing vaccine adjuvants | Novartis AG | 3-
Dec- 09 |
3-
Dec -10 |
28-
Oct- 14 |
| US8871790 | Heterocyclic modulators of lipid synthesis | 3-V Biosciences, Inc. | 8-
Mar- 11 |
8-
Mar -12 |
28-
Oct- 14 |
| US8871783 | Substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (cyano- methyl)-amides inhibitors of cathepsin C | Boehringer Ingelheim International GmBh | 14-
Mar- 13 |
12-
Mar -14 |
28-
Oct- 14 |
| US8871782 | Alkoxy substituted imidazoquinolines | 3M Innovative Properties Company | 3-
Oct- 03 |
1-
Oct -04 |
28-
Oct- 14 |
| US8871503 | Construct | Isis Innovation Limited | 28-
Mar- 06 |
28-
Mar -07 |
28-
Oct- 14 |
| US8871487 | Compositions, methods and uses for inducing viral growth | Takeda Vaccines, Inc. | 5-
Dec- 08 |
4-
Dec -09 |
28-
Oct- 14 |
| US8871442 | Enhanced deposition of chromogens | Ventana Medical Systems, Inc. | 30-
Dec- 10 |
28-
Dec -11 |
28-
Oct- 14 |
| US8865865 | N-terminally modified tetrapeptide derivatives having a C-terminal arginine mimetic | Philipps-Universitat Marburg | 29-
Oct- 08 |
29-
Oct -09 |
21-
Oct- 14 |
| US8865166 | Antibodies to IL-17A and uses thereof | MedImmune Limited | 23-
Jun- 06 |
22-
Jun -07 |
21-
Oct- 14 |
| US8859568 | Pyrrolo[3,2-D]pyrimidin-4-one derivatives and their use in therapy | Astrazeneca AB | 6-
Dec- 04 |
3-
Nov -10 |
14-
Oct- 14 |
| US8859251 | Oligoadenylate synthetase (OAS) | Kineta Two, LLC | 23-
Nov- 05 |
1-
Jul- 13 |
14-
Oct- 14 |
| US8858958 | Adjuvant comprising aluminum, oligonucleotide and polycation | Novartis AG | 27-
Aug- 09 |
27-
Aug -10 |
14-
Oct- 14 |
| US8858957 | GAS57 mutant antigens and GAS57 antibodies | Novartis AG | 12-
Sep- 07 |
14-
Mar -13 |
14-
Oct- 14 |
| US8854617 | Compounds and markers for surface-enhanced Raman scattering | Julius-Maximilians-Universitat Wurzburg | 24-
Sep- 07 |
24-
Sep -08 |
7-
Oct- 14 |
| US8853382 | Expression of antibody or a fragment thereof in lactobacillus | Hera Pharmaceuticals, Inc. | 5-
Aug- 10 |
4-
Aug -11 |
7-
Oct- 14 |
| US8846710 | Method of preferentially inducing the biosynthesis of interferon | 3M Innovative Properties Company | 23-
Feb- 05 |
22-
Feb -06 |
30-
Sep -14 |
| US8846697 | Purine analogs | The Regents of the University of California | 31-
May- 06 |
23-
Apr -07 |
30-
Sep -14 |
| US8846643 | Phosphonates with reduced toxicity for treatment of viral infections | The Regents of the University of California | 14-
Apr- 10 |
11-
Oct -12 |
30-
Sep -14 |
| US8846051 | Modulation of replicative fitness by deoptimization of synonymous codons | The United States of America as represented by the Secretary of the Department of Health and Human Services, Centers for Disease Control and Prevention | 8-
Oct- 04 |
7-
Oct -05 |
30-
Sep -14 |
| US8841100 | Use of methylsulfonylmethane (MSM) to modulate microbial activity | Biogenic Innovations, LLC | 30-
Oct- 09 |
16-
Feb -11 |
23-
Sep -14 |
| US8840899 | Use of mTOR inhibitors to enhance T cell immune responses | Emory University | 5-
Aug- 08 |
5-
Aug -09 |
23-
Sep -14 |
| US8840890 | Rapid expression cloning of human monoclonal antibodies from memory B cells | University of Maryland, Baltimore | 12-
Nov- 08 |
12-
Nov -09 |
23-
Sep -14 |
| US8840873 | Method of treating second and third degree burns using oxidative reductive potential water solution | Oculus Innovative Sciences, Inc. | 23-
Mar- 05 |
23-
Mar -06 |
23-
Sep -14 |
| US8840774 | Electrochemistry and electrogenerated chemiluminescence with a single faradaic electrode | Board of Regents of the University of Texas System | 3-
Jun- 05 |
28-
Oct -13 |
23-
Sep -14 |
| US8835107 | Coronavirus, nucleic acid, protein, and methods for the generation of vaccine, medicaments and diagnostics | Amsterdam Institute of Viral Genomics B.V. | 18-
Aug- 03 |
26-
Jul- 10 |
16-
Sep -14 |
| US8834445 | Methods of treating or preventing peritonitis with oxidative reductive potential water solution | Oculus Innovative Sciences, Inc. | 20-
Jan- 06 |
30-
Mar -12 |
16-
Sep -14 |
| US8828962 | SiRNA compositions and methods for potently inhibiting viral infection | Xiangxue Group (Hong Kong) Company Limited | 11-
Dec- 08 |
12-
Dec -11 |
9-
Sep -14 |
| US8828956 | Carbohydrate conjugates as delivery agents for oligonucleotides | Alnylam Pharmaceuticals, Inc. | 4-
Dec- 07 |
4-
Dec -12 |
9-
Sep -14 |
| US8828940 | Method of treating an ischemia-reperfusion injury-related disorder by administering GPCR ligands | Compugen Ltd. | 18-
Sep- 06 |
14-
Dec -10 |
9-
Sep -14 |
| US8828929 | Cytotoxic T cell epitope peptide for SARS coronavirus, and use thereof | Japan as Represented by Director-General of National Institute of Infectious Diseases | 28-
Nov- 08 |
27-
Nov -09 |
9-
Sep -14 |
| US8828673 | Mixed cell diagnostic systems for detection of respiratory, herpes and enteric viruses | Diagnostic Hybrids Inc | 24-
Apr- 98 |
1-
Mar -12 |
9-
Sep -14 |
| US8828659 | Method for producing nucleic acid probes | Ventana Medical Systems, Inc. | 1-
Sep- 06 |
11-
Mar -13 |
9-
Sep -14 |
| US8828407 | Chimaeric protein | The Pirbright Institute | 7-Jul-
09 |
5-
Jul- 10 |
9-
Sep -14 |
| US8828406 | Influenza viruses and uses thereof | Icahn School of Medicine at Mount Sinai | 30-
Jul-09 |
29-
Jul- 10 |
9-
Sep -14 |
| US8822512 | Crystalline tripeptide epoxy ketone protease inhibitors | Onyx Therapeutics, Inc. | 20-
Mar- 09 |
20-
Sep -11 |
2-
Sep -14 |
| US8822409 | Compositions and uses thereof for the treatment of acute respiratory distress syndrome (ARDS) and clinical disorders associated with therewith | Phylogica Limited | 20-
Jun- 07 |
20-
Jun -08 |
2-
Sep -14 |
| US8821897 | Viral adjuvants | The University of North Carolina at Chapel Hill | 9-Jul-
04 |
24-
Nov -10 |
2-
Sep -14 |
| US8816089 | Methods for controlling SR protein phosphorylation, and antiviral agents whose active ingredients comprise agents that control SR protein activity | Masatoshi Hagiwara | 26-
Dec- 03 |
19-
Nov -12 |
26-
Aug -14 |
| US8816053 | Methods for treating viral infection using IL-28 and IL-29 cysteine mutants | ZymoGenetics, Inc. | 2-
Apr- 04 |
7-
Sep -12 |
26-
Aug -14 |
| US8815837 | Respiratory disease treatment | Pulmagen Therapeutics (Inflammation) Limited | 7-
Aug- 08 |
29-
Jun -12 |
26-
Aug -14 |
| US8815831 | Treatment of Acinetobacter with alginate oligomers and antibiotics | Algipharma AS | 3-
Jun- 09 |
3-
Jun -10 |
26-
Aug -14 |
| US8815611 | Surface for label independent detection and method thereof | Corning Incorporated | 10-
Apr- 08 |
3-
Apr -09 |
26-
Aug -14 |
| US8815249 | Ii-key/antigenic epitope hybrid peptide vaccines | Antigen Express, Inc. | 4-
Sep- 99 |
26-
Jul- 10 |
26-
Aug -14 |
| US8815244 | Method for production of antibody using ostrich | Japan Science and Technology Agency | 29-
Aug- 05 |
16-
Aug -11 |
26-
Aug -14 |
| US8809377 | Deubiquitinase inhibitors and methods for use of the same | The Regents of the University of Michigan | 24-
Sep- 10 |
23-
Sep -11 |
19-
Aug -14 |
| US8808703 | Compounds (cystein based lipopeptides) and compositions as TLR2 agonists used for treating infections, inflammations, respiratory diseases etc | Not Available | 23-
Mar- 10 |
23-
Mar -11 |
19-
Aug -14 |
| US8808686 | Adjuvant-sparing multi-dose influenza vaccination regimen | Novartis AG | 15-
Jun- 06 |
19-
Sep -11 |
19-
Aug -14 |
| US8802853 | Arylalkenyl and arylalkynyl substituted imidazoquinolines | 3M Innovative Properties Company | 29-
Dec- 03 |
17-
Dec -04 |
12-
Aug -14 |
| US8802647 | Materials and methods for prevention and treatment of RNA viral diseases | University of South Florida | 30-
Apr- 02 |
17-
Sep -12 |
12-
Aug -14 |
| US8802106 | Peptide compositions and methods for inhibiting herpesvirus infection | The Administrators of the Tulane Educational Fund | 30-
Oct- 09 |
29-
Oct -10 |
12-
Aug -14 |
| US8796423 | Anti-TSG101 antibodies and their uses for treatment of viral infections | Eli Lilly and Company | 15-
Nov- 06 |
18-
Apr -08 |
5-
Aug -14 |
| US8790655 | Conjugates of synthetic TLR agonists and uses therefor | The Regents of The University of California | 7-
Feb- 07 |
8-
Jan -13 |
29-
Jul- 14 |
| US8785408 | Compositions and methods for reducing or protecting against delayed graft function (DGF) | Quark Pharmaceuticals, Inc. | 27-
Jun- 07 |
26-
Jun -08 |
22-
Jul- 14 |
| US8785375 | Cyclic antimicrobial peptides for treating bacterial infections | Novabiotics Ltd. | 22-
Dec- 05 |
22-
Aug -12 |
22-
Jul- 14 |
| US8784900 | Antimicrobial solutions containing dichlorine monoxide and methods of making and using the same | Oculus Innovative Sciences, Inc. | 13-
Mar- 07 |
13-
Mar -08 |
22-
Jul- 14 |
| US8779132 | Pharmaceutical compounds | Astex Therapeutics Limited | 12-
Oct- 06 |
12-
Oct -07 |
15-
Jul- 14 |
| US8778963 | Hydroxylamine and oxime substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines | 3M Innovative Properties Company | 25-
Nov- 03 |
24-
Nov -04 |
15-
Jul- 14 |
| US8778846 | Composition, device and associated method | General Electric Company | 4-
Dec- 06 |
1-
Mar -07 |
15-
Jul- 14 |
| US8778845 | Composition, device and associated method | Genral Electric Company | 15-
Dec- 05 |
1-
Mar -07 |
15-
Jul- 14 |
| US8778358 | Immunogenic compositions for gram positive bacteria such as Streptococcus agalactiae | Novartis Vaccines and Diagnostics, Inc. | 29-
Jul-04 |
18-
Oct -10 |
15-
Jul- 14 |
| US8778275 | Methods for producing vaccine adjuvants | Novartis AG | 3-
Dec- 09 |
3-
Dec -10 |
15-
Jul- 14 |
| US8772471 | Targeted delivery of siRNA | Immune Disease Institute | 26-
Jan- 07 |
25-
Jan -08 |
8-
Jul- 14 |
| US8765939 | Pyrimidline derivatives having immune modulating properties that act via TLR7 for the treatment of viral or allergic diseases and cancers | AstraZeneca AB | 22-
Nov- 07 |
16-
Aug -12 |
1-
Jul- 14 |
| US8765704 | Modified small interfering RNA molecules and methods of use | Novartis AG | 28-
Feb- 08 |
14-
Dec -11 |
1-
Jul- 14 |
| US8765643 | Composition, device and associated method | General Electric Company | 4-
Dec- 06 |
1-
Mar -07 |
1-
Jul- 14 |
| US8765146 | Adenoviral vector-based malaria vaccines | GenVec, Inc. | 31-
Aug- 05 |
31-
Aug -06 |
1-
Jul- 14 |
| US8765138 | Antiviral and antibacterial activity from medicinal mushrooms | Not Available | 6-
Jan- 04 |
24-
Sep -08 |
1-
Jul- 14 |
| US8765133 | Method of producing anti-CD166 antibody in ostrich | Japan Science and Technology Agency | 29-
Aug- 05 |
16-
Aug -11 |
1-
Jul- 14 |
| US8759307 | Oligonucleotide compound and method for treating nidovirus infections | Sarepta Therapeutics, Inc. | 24-
Dec- 03 |
25-
Apr -08 |
24-
Jun- 14 |
| US8758763 | Archaeal polar lipid aggregates for administration to animals | National Research Council of Canada | 15-
Dec- 06 |
23-
Jan -13 |
24-
Jun- 14 |
| US8758680 | Method and device for cleaning air | Not Available | 29-
Sep- 10 |
27-
Sep -11 |
24-
Jun- 14 |
| US8754071 | Compounds and compositions as c-kit kinase inhibitors | Not Available | 1-
Sep- 11 |
19-
Sep -13 |
17-
Jun- 14 |
| US8754015 | Modified phage for displaying post-translationally modified proteins and uses thereof | University of Rochester | 21-
Nov- 06 |
20-
Nov -07 |
17-
Jun- 14 |
| US8748567 | Method for delivery across the blood brain barrier | Children’s Medical Center Corporation | 22-
May- 06 |
22-
May -07 |
10-
Jun- 14 |
| US8748464 | Use of SIRT1 activators or inhibitors to modulate an immune response | The J. David Gladstone Institutes | 7-
Feb- 08 |
16-
Jul- 10 |
10-
Jun- 14 |
| US8748405 | Methods and compositions for the treatment of cancer or other diseases | City of Hope | 26-
Jan- 07 |
9-
Sep -11 |
10-
Jun- 14 |
| US8748156 | Animal protein-free media for cultivation of cells | Baxter Healthcare SA | 29-
Oct- 04 |
16-
Apr -13 |
10-
Jun- 14 |
| US8741813 | Composition, device and associated method | General Electric Company | 15-
Dec- 05 |
28-
Feb -07 |
3-
Jun- 14 |
| US8741653 | Single recombination system and methods of use | Emergent Product Development GmbH | 22-
Dec- 08 |
16-
Dec -09 |
3-
Jun- 14 |
| US8741604 | Nucleic acid molecule encoding a specific IL-1R1 antibody | Medimmune Limited | 7-
Nov- 08 |
14-
Sep -12 |
3-
Jun- 14 |
| US8741564 | Quantitative nuclease protection assay (QNPA) and sequencing (QNPS) improvements | HTG Molecular Diagnostics, Inc. | 4-
May- 11 |
26-
Apr -12 |
3-
Jun- 14 |
| US8741311 | Methods and compositions for immunization against virus | Academia Sinica | 27-
Mar- 09 |
26-
Mar -10 |
3-
Jun- 14 |
| US8735567 | Multi-targeted RNAi therapeutics for scarless wound healing of skin | Not Available | 6-
Nov- 07 |
6-
Nov -08 |
27-
May -14 |
| US8735559 | Mutant protease biosensors with enhanced detection characteristics | Promega Corporation | 11-
May- 10 |
11-
May -11 |
27-
May -14 |
| US8735421 | Imidazoquinolinyl sulfonamides | 3M Innovative Properties Company | 30-
Dec- 03 |
23-
Dec -04 |
27-
May -14 |
| US8735410 | Quinazoline derivatives as tyrosine kinase inhibitors | AstraZeneca AB | 26-
Feb- 05 |
24-
Feb -06 |
27-
May -14 |
| US8735348 | Casein derived peptides and uses thereof | Peptera Ltd. | 1-
Mar- 00 |
5-
Sep -12 |
27-
May -14 |
| US8734823 | Device including altered microorganisms, and methods and systems of use | The Invention Science Fund I, LLC | 14-
Dec- 05 |
28-
May -10 |
27-
May -14 |
| US8728793 | Amphipathic alpha-helical peptide compositions as antiviral agents | The Board of Trustees of the Leland Stanford Junior University | 19-
Jul-07 |
14-
Jul- 08 |
20-
May -14 |
| US8722917 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
23-
Jan -12 |
13-
May -14 |
| US8722741 | Biphenyloxyacetic acid derivatives for the treatment of respiratory disease | AstraZeneca AB | 24-
Aug- 04 |
6-
Dec -11 |
13-
May -14 |
| US8722725 | Caffeoylquinic acid derivatives containing nitrogen, and preparation method, pharmaceutical composition and usage thereof | Zhejiang Medicine Co., Ltd. Xinchang Pharmaceutical Factory | 23-
Mar- 07 |
21-
Mar -08 |
13-
May -14 |
| US8718948 | Systems and methods for distinguishing optical signals of different modulation frequencies in an optical signal detector | Gen-Probe Incorporated | 24-
Feb- 11 |
24-
Feb -12 |
6-
May -14 |
| US8716464 | Compositions and methods for silencing Ebola virus gene expression | Not Available | 20-
Jul-09 |
20-
Jul- 10 |
6-
May -14 |
| US8716461 | Human parvovirus | Blood Systems, Inc. | 24-
May- 04 |
24-
May -05 |
6-
May -14 |
| US8710224 | Heterocyclic compounds as CCR2B antagonists | AstraZeneca AB | 24-
Dec- 04 |
13-
Sep -13 |
29-
Apr- 14 |
| US8709730 | Methods of preventing and treating viral infections by inhibiting the deISGylation activity of OTU domain-containing viral proteins | Icahn School of Medicine at Mount Sinai | 5-
Apr- 07 |
7-
Apr -08 |
29-
Apr- 14 |
| US8709496 | Use of deuterium oxide for the treatment of virus-based diseases of the respiratory tract | D2 Bioscience Group Ltd. | 6-
Jan- 10 |
23-
May -12 |
29-
Apr- 14 |
| US8709447 | Compositions and methods for activating innate and allergic immunity | ID Biomedical Corporation of Quebec | 22-
Oct- 03 |
26-
Apr -12 |
29-
Apr- 14 |
| US8709441 | TC-83-derived alphavirus vectors, particles and methods | Alphavax, Inc. | 18-
May- 04 |
6-
Jul- 10 |
29-
Apr- 14 |
| US8704169 | Direct impact ionization (DII) mass spectrometry | The United States of America, as represented by the Secretary, Department of Health and Human Services | 11-
Oct- 11 |
11-
Oct -11 |
22-
Apr- 14 |
| US8703748 | Cleaning composition for treating tissue for transplantation derived from human/animal | CG BIO Co., Ltd. | 11-
Feb- 09 |
10-
Feb -10 |
22-
Apr- 14 |
| US8703467 | Inactivation of a pathogen in a sample by a treatment with formalin and UV light | Baxter Healthcare SA | 27-
May- 04 |
26-
May -05 |
22-
Apr- 14 |
| US8702958 | Electrochemistry and electrogenerated chemiluminescence with a single faradaic electrode | Board of Regents of the University of Texas System | 3-
Jun- 05 |
31-
May -12 |
22-
Apr- 14 |
| US8697873 | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines | 3M Innovative Properties Company | 24-
Mar- 04 |
24-
Mar -05 |
15-
Apr- 14 |
| US8697853 | TAL effector-mediated DNA modification | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
10-
Jan -13 |
15-
Apr- 14 |
| US8697659 | Analogues of glycolipids useful as immunoadjuvants | Luigi Panza | 12-
Oct- 07 |
10-
Oct -08 |
15-
Apr- 14 |
| US8697140 | Virucidal disinfectant | B. Braun Medical AG | 28-
Jan- 05 |
28-
May -09 |
15-
Apr- 14 |
| US8697088 | VLPs derived from cells that do not express a viral matrix or core protein | Novavax, Inc. | 25-
May- 07 |
27-
May -08 |
15-
Apr- 14 |
| US8697087 | Influenza vaccines including combinations of particulate adjuvants and immunopotentiators | Novartis AG | 4-
Nov- 05 |
6-
Nov -06 |
15-
Apr- 14 |
| US8691837 | Substituted imidazo ring systems and methods | 3M Innovative Properties Company | 25-
Nov- 03 |
24-
Nov -04 |
8-
Apr- 14 |
| US8691826 | Compounds | Chiesi Farmaceutici S.p.A. | 14-
Sep- 11 |
13-
Sep -12 |
8-
Apr- 14 |
| US8691781 | Compositions for treating respiratory viral infections and their use | Sirnaomics, Inc. | 5-
Nov- 04 |
4-
Nov -05 |
8-
Apr- 14 |
| US8691777 | Combination therapy | Emory University | 27-
Jan- 11 |
25-
Jan -12 |
8-
Apr- 14 |
| US8686152 | 4,4-disubstituted piperidine derivatives useful as inhibitors of dipeptidyl peptidase-1 (DPP-1) | Janssen Pharmaceutica NV | 10-
Mar- 10 |
9-
Mar -11 |
1-
Apr- 14 |
| US8682619 | Device including altered microorganisms, and methods and systems of use | The Invention Science Fund I, LLC | 14-
Dec- 05 |
28-
May -10 |
25-
Mar -14 |
| US8679839 | Cell line from rousettus as host cell for pathogen amplification | Probiogen AG | 4-
Mar- 08 |
4-
Mar -09 |
25-
Mar -14 |
| US8678184 | Methods for producing vaccine adjuvants | Novartis AG | 3-
Dec- 09 |
3-
Dec -10 |
25-
Mar -14 |
| US8678002 | Devices and methods for decreasing human pathogen transmission | Filligent Limited | 26-
Jun- 07 |
25-
Jun -08 |
25-
Mar -14 |
| US8673983 | Melanins synthesized chemically or via enzyme catalysis | Loyola University Chicago | 21-
Dec- 07 |
22-
Dec -08 |
18-
Mar -14 |
| US8673932 | Oxime substituted imidazo-containing compounds | 3M Innovative Properties Company | 12-
Aug- 03 |
12-
Aug -04 |
18-
Mar -14 |
| US8673907 | Pharmaceutically acceptable salts of methyl (3-{ [[3-(6-amino- 2- butoxy-8-oxo-7,8-dihydro-9H-purin-9-yl) propyl] (3-morpholin-4- ylpropyl) amino] methyl }phenyl) acetate and their use in therapy | AstraZeneca AB | 17-
Dec- 07 |
16-
Dec -08 |
18-
Mar -14 |
| US8673904 | Epoxide inhibitors of cysteine proteases | The Board of Trustees of the Leland Stanford Junior University | 13-
Jun- 06 |
13-
Jun -07 |
18-
Mar -14 |
| US8673558 | Luciferase biosensor | Promega Corporation | 10-
Oct- 03 |
24-
Apr -12 |
18-
Mar -14 |
| US8673331 | Composition with sterilizing activity against bacteria, fungus and viruses, application thereof and method for preparation thereof | GP&E | 18-
Nov- 11 |
18-
Nov -11 |
18-
Mar -14 |
| US8669263 | Use of TAM receptor inhibitors as antimicrobials | Salk Institute for Biological Studies | 9-
Nov- 07 |
8-
Mar -13 |
11-
Mar -14 |
| US8669262 | 3,5-diamino-6-chloro-N-(N-(4-(4-(2-(hexyl(2,3,4,5,6-
pentahydroxyhexyl)amino)ethoxy)phenyl)butyl)carbamimidoyl)pyra zine-2-carboxamide |
Parion Sciences, Inc. | 27-
Jun- 11 |
26-
Jun -12 |
11-
Mar -14 |
| US8669240 | Biological specimen collection and transport system and method of use | Longhorn Vaccines & Diagnostics, LLC | 1-
Oct- 07 |
19-
Mar -13 |
11-
Mar -14 |
| US8664274 | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arena viruses | Siga Technologies, Inc. | 6-
Dec- 04 |
6-
Jul- 11 |
4-
Mar -14 |
| US8664218 | Pharmaceutical compounds | Astex Therapeutics Ltd. | 11-
Apr- 08 |
30-
Jan -13 |
4-
Mar -14 |
| US8664188 | siRNA compositions and methods for potently inhibiting viral infection | Xiangxue Group (Hong Kong) Company Limited | 11-
Dec- 08 |
11-
Dec -09 |
4-
Mar -14 |
| US8663922 | Systems and methods for detecting multiple optical signals | Gen-Probe Incorporated | 10-
Mar- 05 |
1-
Jun -10 |
4-
Mar -14 |
| US8658767 | Lipidated polyepitope vaccines | National Health Research Institutes | 15-
Nov- 10 |
15-
Nov -11 |
25-
Feb -14 |
| US8658697 | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses | Siga Technologies, Inc. | 6-
Dec- 04 |
26-
Oct -10 |
25-
Feb -14 |
| US8658666 | Substituted imidazoquinolines and imidazonaphthyridines | 3M Innovative Properties Company | 11-
Feb- 05 |
10-
Feb -06 |
25-
Feb -14 |
| US8658178 | Carbon nanotube compositions and methods of use thereof | Yale University | 19-
Mar- 08 |
19-
Mar -09 |
25-
Feb -14 |
| US8653252 | Short interfering RNA (siRNA) analogues | Santaris Pharma A/S | 21-
Mar- 03 |
22-
Mar -04 |
18-
Feb -14 |
| US8653084 | Hydrobenzamide derivatives as inhibitors of Hsp90 | Astex Therapeutics Ltd. | 12-
Oct- 06 |
12-
Oct -07 |
18-
Feb -14 |
| US8653034 | Compositions and methods comprising phosphatidylethanolaminebinding peptide derivatives | Board of Regents, The University of Texas System | 15-
Jul-02 |
8-
May -08 |
18-
Feb -14 |
| US8652836 | Defective ribosomal products in blebs (DRibbles) and methods of use to stimulate an immune response | Providence Health System | 29-
Jul-05 |
27-
Jul- 06 |
18-
Feb -14 |
| US8652782 | Compositions and methods for detecting, identifying and quantitating mycobacterial-specific nucleic acids | Longhorn Vaccines & Diagnostics, LLC | 12-
Sep- 06 |
26-
Apr -11 |
18-
Feb -14 |
| US8652533 | Durable biocides and disinfectants | Mitsui Norin Co., Ltd. | 7-Jul-
04 |
5-
Jul- 05 |
18-
Feb -14 |
| US8648076 | Cysteine protease inhibitors and their therapeutic applications | Hybrigenics SA | 5-
Aug- 05 |
26-
Jul- 06 |
11-
Feb -14 |
| US8647676 | Antimicrobial composition from copepods | Nofima Ingrediens | 28-
Oct- 08 |
28-
Oct -09 |
11-
Feb -14 |
| US8642596 | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arena viruses | Siga Technologies, Inc. | 6-
Dec- 04 |
6-
Dec -05 |
4-
Feb -14 |
| US8642260 | Single quantum-dot based aptameric nanosensors | The Research Foundation of the City University of New York | 21-
Oct- 08 |
21-
Oct -09 |
4-
Feb -14 |
| US8633322 | Alkynyl derivatives useful as DPP-1 inhibitors | Janssen Pharmaceutica NV | 29-
Oct- 09 |
28-
Oct -10 |
21-
Jan- 14 |
| US8633308 | Compounds for preventing or treating viral infections and methods of use thereof | The Governors of The University of Alberta | 28-
Feb- 07 |
27-
Feb -08 |
21-
Jan- 14 |
| US8632764 | Directed evolution and in vivo panning of virus vectors | University of North Carolina at Chapel Hill | 30-
Apr- 08 |
29-
Apr -09 |
21-
Jan- 14 |
| US8629283 | Compounds that modulate negative-sense, single-stranded RNA virus replication and uses thereof | Icahn School of Medicine at Mount Sinai | 6-
Mar- 08 |
6-
Mar -09 |
14-
Jan- 14 |
| US8629271 | Compounds | AstraZeneca AB | 6-
Feb- 08 |
2-
Apr -12 |
14-
Jan- 14 |
| US8629098 | Compositions and methods for adoptive and active immunotherapy | Yale University | 15-
Jan- 08 |
14-
Jan -09 |
14-
Jan- 14 |
| US8628786 | Polychlorinated biphenyls and squalene-containing adjuvants | Novartis AG | 28-
Dec- 07 |
2-
Dec -11 |
14-
Jan- 14 |
| US8624011 | Vaccines and immunotherapeutics comprising IL-15 receptor alpha and/or nucleic acid molecules encoding the same, and methods for using the same | The Trustees of the University of Pennsylvania | 14-
Sep- 09 |
14-
Sep -10 |
7-
Jan- 14 |
| US8623419 | Technology for preparation of macromolecular microspheres | Ansun Biopharma, Inc. | 24-
Jan- 06 |
4-
Nov -11 |
7-
Jan- 14 |
| US8623382 | Immunogenic compositions for inducing an immune response to HIV | Wyeth LLC | 17-
Jun- 04 |
18-
Jan -11 |
7-
Jan- 14 |
| US8623364 | Antigenic GM-CSF peptides and antibodies to GM-CSF | Morphotek, Inc. | 8-
Feb- 06 |
24-
Oct -12 |
7-
Jan- 14 |
| US8617838 | Fluorescent proteins and related methods and compounds | University of Massachusetts | 20-
Sep- 04 |
20-
Sep -05 |
31-
Dec -13 |
| US8615368 | Method for determining the amount of an analyte in a sample | Gen-Probe Incorporated | 10-
Mar- 05 |
10-
Mar -06 |
24-
Dec -13 |
| US8609370 | Highly active glycoproteins-process conditions and an efficient method for their production | Glycotope GmbH | 13-
Feb- 04 |
14-
Feb -05 |
17-
Dec -13 |
| US8609101 | Granulocyte-macrophage colony-stimulating factor (GM-CSF) neutralizing antibodies | Theraclone Sciences, Inc. | 23-
Apr- 09 |
23-
Apr -10 |
17-
Dec -13 |
| US8604215 | Crystalline tripeptide epoxy ketone protease inhibitors | Onyx Therapeutics, Inc. | 20-
Mar- 09 |
22-
Mar -10 |
10-
Dec -13 |
| US8603469 | Methods of treating cancer with human monoclonal antibodies against interleukin 8 | Genmab A/S | 16-
Dec- 02 |
27-
Dec -11 |
10-
Dec -13 |
| US8599383 | Optical cytometry | The Regents of the University of California | 6-
May- 09 |
6-
May -09 |
3-
Dec -13 |
| US8598192 | Hydroxylamine substituted imidazoquinolines | 3M Innovative Properties Company | 14-
Nov- 03 |
12-
Nov -04 |
3-
Dec -13 |
| US8598134 | RNAi modulation of RSV, PIV and other respiratory viruses and uses thereof | South Alabama Medical Science Foundation | 22-
Oct- 04 |
23-
Jul- 10 |
3-
Dec -13 |
| US8598116 | Treatment of influenza virus infection | Educational Fund and Autoimmune Technologies, LLC | 4-
Nov- 03 |
29-
May -12 |
3-
Dec -13 |
| US8598106 | Anti-microbial composition exhibiting residual anti-microbial properties on a surface | Byotrol PLC | 17-
Sep- 07 |
5-
Jul- 11 |
3-
Dec -13 |
| US8597650 | Methods for treating rheumatoid arthritis with anti-bile salt- stimulated lipase (BSSL) antibodies | HERNELL OLLE | 8-
Apr- 09 |
6-
Apr -10 |
3-
Dec -13 |
| US8592567 | Vaccines and immunotherapeutics using codon-optimized IL-15 and methods for using the same | The Trustees of the University of Pennsylvania | 13-
Jan- 06 |
3-
May -12 |
26-
Nov -13 |
| US8592391 | Method for therapeutic, clinical and veterinary use poly-ICLC | SALAZAR ANDRES | 1-Jul-
03 |
17-
Oct -08 |
26-
Nov -13 |
| US8586770 | Unsaturated steroid compounds | Harbor Therapeutics, Inc. | 29-
Sep- 04 |
18-
Feb -11 |
19-
Nov -13 |
| US8586364 | Cells and methodology to generate non-segmented negative-strand RNA viruses | Institut Pasteur | 22-
Dec- 06 |
21-
Dec -07 |
19-
Nov -13 |
| US8586363 | TAL effector-mediated DNA modification | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
10-
Dec -10 |
19-
Nov -13 |
| US8581584 | Membrane proteins, mechanisms of action and uses thereof | Florida State University Research Foundation | 26-
May- 10 |
26-
May -11 |
12-
Nov -13 |
| US8580927 | Engineered antibody constant domain molecules | The United States of America, as represented by the Secretary, Department of Health and Human Services | 31-
Jan- 08 |
30-
Jan -09 |
12-
Nov -13 |
| US8580268 | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity | Coley Pharmaceutical GmbH | 27-
Sep- 06 |
27-
Sep -07 |
12-
Nov -13 |
| US8569283 | Compounds and compositions as c-Kit kinase inhibitors | LIU XIAODONG | 1-
Sep- 11 |
29-
Aug -12 |
29-
Oct- 13 |
| US8562996 | RSV-specific binding molecules and means for producing them | MedImmune Limited | 1-
Jun- 07 |
30-
May -08 |
22-
Oct- 13 |
| US8562943 | Quality control methods for oil-in-water emulsions containing squalene | Novartis AG | 8-
Nov- 06 |
6-
Nov -07 |
22-
Oct- 13 |
| US8560339 | System and method to predict the global spread of infectious agents via commercial air travel | Kamran Khan | 2-
Apr- 07 |
2-
Apr -08 |
15-
Oct- 13 |
| US8557767 | Synthetic apolipoprotein E mimicking polypeptides and methods of use | UAB Research Foundation | 28-
Aug- 07 |
27-
Aug -08 |
15-
Oct- 13 |
| US8557248 | Methods and compositions for treating malaria | Cyvax, Inc. | 9-
Aug- 10 |
9-
Aug -11 |
15-
Oct- 13 |
| US8552051 | Use of pharmaceutical compositions containing mesembrenone | H. L. Hall & Sons Limited | 20-
Mar- 09 |
16-
Mar -10 |
8-
Oct- 13 |
| US8552032 | Bicyclic derivatives useful as inhibitors of DPP-1 | Janssen Pharmaceutica NV | 18-
Dec- 09 |
16-
Dec -10 |
8-
Oct- 13 |
| US8551968 | Methods for generation of antibodies | National Jewish Health | 13-
Mar- 07 |
13-
Mar -08 |
8-
Oct- 13 |
| US8551756 | Avian influenza chimeric VLPS | Novavax, Inc. | 11-
Jul-03 |
19-
Jan -10 |
8-
Oct- 13 |
| US8551750 | Device including bone cage and method for treatment of disease in a subject | The Invention Science Fund I, LLC | 23-
Apr- 09 |
28-
Jul- 09 |
8-
Oct- 13 |
| US8551749 | Device including bone cage and method for treatment of disease in a subject | The Invention Science Fund I, LLC | 23-
Apr- 09 |
23-
Apr -09 |
8-
Oct- 13 |
| US8551738 | Systems and methods for rapid identification of nucleic acid variants | Ibis Biosciences, Inc. | 21-
Jul-05 |
11-
Nov -09 |
8-
Oct- 13 |
| US8551469 | Treatment of tumors and viral diseases with recombinant interferon alpha | Superlab Far East Limited | 28-
Feb- 01 |
6-
Oct -08 |
8-
Oct- 13 |
| US8546432 | Tetrazolones as inhibitors of fatty acid synthase | Infinity Pharmaceuticals, Inc. | 5-
May- 10 |
5-
May -11 |
1-
Oct- 13 |
| US8546383 | Chiral fused [1,2]imidazo[4,5-c] ring compounds | 3M Innovative Properties Company | 30-
Dec- 04 |
25-
May -12 |
1-
Oct- 13 |
| US8546082 | Methods for identification of sepsis-causing bacteria | Ibis Biosciences, Inc. | 11-
Sep- 03 |
25-
May -07 |
1-
Oct- 13 |
| US8541568 | Compositions and methods using siRNA molecules for treatment of gliomas | BIGNER DARELL D | 24-
May- 08 |
26-
May -09 |
24-
Sep -13 |
| US8541457 | Aminothiazole derivatives as human stearoyl-CoA desaturase inhibitors | Xenon Pharmaceuticals Inc. | 3-
Jun- 05 |
5-
Jun -06 |
24-
Sep -13 |
| US8541438 | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines | 3M Innovative Properties Company | 18-
Jun- 04 |
21-
Dec -10 |
24-
Sep -13 |
| US8541221 | Primate T-lymphotropic viruses | Johns Hopkins University | 21-
Feb- 05 |
1-
Jul- 10 |
24-
Sep -13 |
| US8541003 | Vectors expressing SARS immunogens, compositions containing such vectors or expression products thereof, methods and assays for making and using | Protein Sciences Corporation | 20-
Jun- 03 |
21-
Jun -04 |
24-
Sep -13 |
| US8529968 | Decontaminating composition having simultaneously bactericidal, fungicidal and virocidal properties, methods for obtaining and using said composition | Hightech Bio-Activities Holding GmbH | 29-
Mar- 04 |
29-
Mar -05 |
10-
Sep -13 |
| US8524715 | Phenoxyacetic acid derivatives useful for treating respiratory diseases | Astrazeneca AB | 23-
Nov- 04 |
22-
Nov -05 |
3-
Sep -13 |
| US8524488 | Methods and devices for determining a cell characteristic, and applications employing the same | The Regents of the University of California | 10-
Sep- 02 |
9-
Mar -05 |
3-
Sep -13 |
| US8524241 | Fusion proteins comprising a fragment of Vibrio cholerae exotoxin A | The General Hospital Corporation | 20-
Jul-07 |
18-
Jul- 08 |
3-
Sep -13 |
| US8519106 | Monoclonal human tumor-specific antibody | University of Zurich | 13-
Mar- 07 |
13-
Mar -08 |
27-
Aug -13 |
| US8507545 | Cytotoxic T cell activator comprising EP4 agonist | National University Corporation, Hamamatsu University School of Medicine | 8-
May- 07 |
7-
May -08 |
13-
Aug -13 |
| US8507544 | Bi-aryl amide compounds as CRTh2 receptor modulators | Astrazeneca AB | 5-Jul-
07 |
3-
Jul- 08 |
13-
Aug -13 |
| US8507455 | Folate conjugates | Alnylam Pharmaceuticals, Inc. | 4-
Dec- 07 |
4-
Dec -08 |
13-
Aug -13 |
| US8506968 | SARS vaccine compositions and methods of making and using them | Eli Lilly and Company | 29-
Jun- 00 |
28-
Dec -09 |
13-
Aug -13 |
| US8506966 | Adjuvanted influenza vaccines for pediatric use | Novartis AG | 22-
Feb- 08 |
20-
Feb -09 |
13-
Aug -13 |
| US8501746 | Organic compounds | Novartis AG | 5-
Jun- 06 |
4-
Jun -07 |
6-
Aug -13 |
| US8501699 | Bicyclic nucleosides and nucleotides as therapeutic agents | Biota Scientific Management Pty Ltd | 3-Jul-
08 |
7-
Sep -12 |
6-
Aug -13 |
| US8501461 | System for performing multi-formatted assays | Gen-Probe Incorporated | 10-
Mar- 05 |
3-
Dec -09 |
6-
Aug -13 |
| US8497405 | Process for dispersing vaporous hydrogen peroxide | STERIS Inc. | 6-
Mar- 07 |
28-
Feb -13 |
30-
Jul- 13 |
| US8497112 | Method for producing viral vaccines | Baxter Healthcare SA | 28-
Aug- 07 |
28-
Aug -08 |
30-
Jul- 13 |
| US8494781 | Systems and methods for identifying replikin scaffolds and uses of said replikin scaffolds | BOGOCH ELENORE S | 6-
Jun- 03 |
10-
Dec -10 |
23-
Jul- 13 |
| US8492329 | Bioactive peptides and methods of using same | Compugen Ltd. | 12-
Jul-07 |
11-
Jul- 08 |
23-
Jul- 13 |
| US8486959 | Dibenzo[f,h]isoquinoline derivatives | National Health Research Institutes | 14-
Jan- 10 |
14-
Jan -11 |
16-
Jul- 13 |
| US8486678 | Pharmaceutical compositions for the treatment of virus infection | Kineta Two, LLC | 23-
Nov- 05 |
1-
Feb -12 |
16-
Jul- 13 |
| US8486619 | Arrayed imaging reflectometry (air) sensor chip comprising influenza hemagglutinin (HA) polypeptides suitable for the detection of antiviral immune responses | University of Rochester | 2-
May- 08 |
1-
May -09 |
16-
Jul- 13 |
| US8486420 | Live virus vaccines | Children’s Hospital, Inc. | 15-
Feb- 05 |
15-
Feb -06 |
16-
Jul- 13 |
| US8481547 | Substituted benzothiazole and benzoxazole derivatives useful as inhibitors of DPP-1 | Janssen Pharmaceutica NV | 18-
Dec- 09 |
16-
Dec -10 |
9-
Jul- 13 |
| US8481270 | Method for chromogenic detection of two or more target molecules in a single sample | Ventana Medical Systems, Inc. | 22-
Aug- 08 |
21-
Aug -09 |
9-
Jul- 13 |
| US8481255 | Scytovirin domain 1 related polypeptides | The United States of America, as represented by the Secretary, Department of Health and Human Services | 25-
May- 05 |
30-
Sep -11 |
9-
Jul- 13 |
| US8476292 | Amide and carbamate derivatives of N-{2-[4-amino-2- (ethoxymethyl)-1H-imidazo[4,5-c] quinolin-1-Yl]-1,1-
dimethylethyl}methanesulfonamide and methods |
3M Innovative Properties Company | 9-
Sep- 05 |
8-
Sep -06 |
2-
Jul- 13 |
| US8476288 | Salts 756 | AstraZeneca AB | 21-
May- 09 |
20-
May -10 |
2-
Jul- 13 |
| US8476265 | Compounds-801 | AstraZeneca AB | 30-
Jul-10 |
14-
Sep -12 |
2-
Jul- 13 |
| US8470771 | Method and medicament for inhibiting the infection of influenza virus | Institute of Microbiology, Chinese Academy of Sciences | 14-
Nov- 07 |
18-
Dec -07 |
25-
Jun- 13 |
| US8470769 | Method of treatment of bacterial infection by administration of polylysine | Novabiotics, Ltd. | 18-
Aug- 04 |
30-
Nov -11 |
25-
Jun- 13 |
| US8470346 | Anti-viral pharmaceutical compositions | Mast Therapeutics, Inc. | 10-
Dec- 03 |
19-
May -11 |
25-
Jun- 13 |
| US8470335 | Recombinant SARS-CoV nsp12 and the use of thereof and the method for producing it | Industry-Academic Cooperation Foundation, Yonsei University Kookmin University Industry Academy Cooperation Foundation | 13-
Jun- 08 |
13-
Jun -08 |
25-
Jun- 13 |
| US8466284 | Some 2-pyrazinone derivatives and their use as inhibitors of neutrophile elastase | Astra Zeneca AB | 6-
Nov- 07 |
5-
Nov -08 |
18-
Jun- 13 |
| US8466167 | Compounds and compositions as TLR activity modulators | IRM LLC | 3-
Mar- 08 |
27-
Feb -09 |
18-
Jun- 13 |
| US8466124 | RNA sequence motifs in the context of defined internucleotide linkages inducing specific immune modulatory profiles | Coley Pharmaceutical GmbH | 13-
Aug- 07 |
12-
Jun -12 |
18-
Jun- 13 |
| US8465751 | Cna—B domain antigens in vaccines against gram positive bacteria | Novartis AG | 12-
Jan- 09 |
12-
Jan -10 |
18-
Jun- 13 |
| US8461125 | Compositions and methods to treat asthma | The Children’s Hospital of Philadelphia | 14-
Feb- 08 |
13-
Aug -10 |
11-
Jun- 13 |
| US8460914 | Decreasing potential iatrogenic risks associated with vaccines and vaccine antigens | Novartis AG | 9-
Sep- 04 |
10-
Jan -13 |
11-
Jun- 13 |
| US8460605 | Decontaminant dispenser suitable for use as a projectile | STERIS Inc. | 6-
Mar- 07 |
20-
Feb -08 |
11-
Jun- 13 |
| US8455483 | Compounds—801 | AstraZeneca AB | 31-
Jul-09 |
30-
Jul- 10 |
4-
Jun- 13 |
| US8450471 | TAL effector-mediated DNA modification | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
22-
Mar -12 |
28-
May -13 |
| US8450467 | Carbohydrate conjugates as delivery agents for oligonucleotides | Alnylam Pharmaceuticals, Inc. | 4-
Dec- 07 |
14-
Dec -11 |
28-
May -13 |
| US8450350 | Triazoles as inhibitors of fatty acid synthase | Infinity Pharmaceuticals, Inc. | 5-
May- 10 |
5-
May -11 |
28-
May -13 |
| US8450284 | Coiled-coil lipopeptide helical bundles and synthetic virus-like particles | Universitaet Zuerich | 9-
Dec- 06 |
6-
Dec -07 |
28-
May -13 |
| US8450055 | Malaria antigen screening method | The United States of America as Represented by the Secretary of the Navy | 31-
Aug- 05 |
25-
Aug -06 |
28-
May -13 |
| US8445650 | Mutant botulinum neurotoxin serotype A polypeptide and uses thereof | Thomas Jefferson University | 25-
Sep- 07 |
25-
Sep -08 |
21-
May -13 |
| US8445447 | B7-DC variants immunogenic compositions and methods of use thereof | The Johns Hopkins University | 13-
Jul-07 |
7-
Mar -12 |
21-
May -13 |
| US8444961 | RNA virus infection inhibitor, method for inhibition of infection by RNA virus, RNA virus infection-inhibiting product, and use as RNA virus infection inhibitor | Sekisui Chemical Co., Ltd. | 16-
Jun- 09 |
16-
Jun -10 |
21-
May -13 |
| US8440704 | Quercetin-containing compositions | Quercegen Pharmaceuticals LLC | 17-
Jul-06 |
16-
Dec -09 |
14-
May -13 |
| US8440649 | Phenanthroindolizidine analogues | National Health Research Institutes | 24-
Feb- 09 |
11-
Feb -10 |
14-
May -13 |
| US8440642 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
1-
Sep -11 |
14-
May -13 |
| US8440432 | Tal effector-mediated DNA modification | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
22-
Mar -12 |
14-
May -13 |
| US8440431 | TAL effector-mediated DNA modification | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
22-
Mar -12 |
14-
May -13 |
| US8440408 | Animal protein-free media for cultivation of cells | Baxter Healthcare S.A. | 29-
Oct- 04 |
10-
Dec -10 |
14-
May -13 |
| US8436178 | Imidazoquinolines with immuno-modulating properties | AstraZeneca AB | 8-
May- 07 |
6-
May -08 |
7-
May -13 |
| US8436024 | 2-pyridone compounds | Astrazeneca AB | 2-
Oct- 09 |
1-
Oct -10 |
7-
May -13 |
| US8431160 | Microparticles containing biodegradable polymer and cationic polysaccharide for use in immunogenic compositions | Novartis AG | 24-
Feb- 06 |
24-
Feb -07 |
30-
Apr- 13 |
| US8431134 | Use of a pneumococcal P4 peptide for enhancing opsonophagocytosis in response to a pathogen | The United States of America as represented by the Secretary of the Department of Health and Human Services, Centers for Disease Control and Prevention | 31-
Jul-08 |
31-
Jul- 09 |
30-
Apr- 13 |
| US8426565 | Dendritic cell marker and uses thereof | Walter and Eliza Hall Institute of Medical Research | 30-
Aug- 07 |
29-
Aug -08 |
23-
Apr- 13 |
| US8420798 | Method for producing nucleic acid probes | Ventana Medical Systems, Inc. | 1-
Sep- 06 |
31-
Aug -07 |
16-
Apr- 13 |
| US8420784 | Interleukin 10 receptor, (IL-10R) antibodies | Kyowa Hakko Kirin Co., Ltd. | 27-
May- 08 |
27-
May -09 |
16-
Apr- 13 |
| US8420096 | Cell-penetrating SOCS polypeptides that inhibit cytokine-induced signaling | Vanderbilt University | 4-
Mar- 04 |
4-
Mar -05 |
16-
Apr- 13 |
| US8420094 | Fusion proteins comprising a fragment of Vibrio cholerae exotoxin A | The General Hospital Corporation | 20-
Jul-07 |
18-
Jul- 08 |
16-
Apr- 13 |
| US8415394 | Biphenyloxyacetic acid derivatives for the treatment of respiratory disease | Astrazeneca AB | 6-
Oct- 05 |
21-
Dec -11 |
9-
Apr- 13 |
| US8415361 | Use of TAM receptor inhibitors as antimicrobials | The Salk Institute for Biological Studies | 9-
Nov- 07 |
7-
Nov -08 |
9-
Apr- 13 |
| US8415330 | Biological specimen collection and transport system and method of use | Longhorn Vaccines & Diagnostics, LLC | 1-
Oct- 07 |
1-
Oct -12 |
9-
Apr- 13 |
| US8415309 | Bicyclic nucleosides and nucleotides as therapeutic agents | Biota Scientific Managment Pty Ltd | 1-Jul-
09 |
11-
Jan -12 |
9-
Apr- 13 |
| US8415118 | Porcine DC-SIGN, ICAM-3 and LSECtin and uses thereof | Virginia Tech Intellectual Properties, Inc. | 29-
Oct- 07 |
29-
Oct -08 |
9-
Apr- 13 |
| US8415102 | Methods and computer systems for identifying target-specific sequences for use in nanoreporters | NanoString Technologies, Inc. | 10-
Apr- 07 |
10-
Apr -08 |
9-
Apr- 13 |
| US8410149 | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses | Siga Technologies Inc. | 6-
Dec- 04 |
28-
Oct -10 |
2-
Apr- 13 |
| US8410114 | 2-pyrazinone derivatives for the treatment of disease or condition in which inhibition of neutrophil elastase activity is beneficial | AstraZeneca AB | 8-
May- 06 |
6-
Jan -12 |
2-
Apr- 13 |
| US8409589 | Mutant forms of streptolysin O | Novartis AG | 21-
Dec- 07 |
14-
Sep -11 |
2-
Apr- 13 |
| US8399651 | Nucleic acids encoding GAS57 mutant antigens | Novartis AG | 12-
Sep- 07 |
10-
Sep -12 |
19-
Mar -13 |
| US8398992 | Methods and compositions for polytopic vaccination | Polytopos LLC | 29-
Apr- 05 |
22-
Dec -11 |
19-
Mar -13 |
| US8394986 | Phenoxiacetic acid derivatives | AstraZeneca AB | 21-
Aug- 03 |
26-
Jul- 11 |
12-
Mar -13 |
| US8394945 | Compositions for use in identification of bacteria | Ibis Biosciences, Inc. | 11-
Sep- 03 |
7-
Mar -07 |
12-
Mar -13 |
| US8394386 | Sequential delivery of immunogenic molecules via adenovirus and adeno-associated virus-mediated administrations | The Trustees of the University of Pennsylvania | 28-
Apr- 04 |
27-
Apr -05 |
12-
Mar -13 |
| US7829712 | Pyridazine derivatives for inhibiting human stearoyl-CoA-desaturase | Xenon Pharmaceuticals Inc. | 20-
Sep- 04 |
20-
Sep -05 |
9-
Nov -10 |
| US7829707 | Pyrrolo [3,2-d]pyrimidin-4-one derivatives and their use in therapy | AstraZeneca AB | 6-
Dec- 04 |
5-
Dec -05 |
9-
Nov -10 |
| US7829302 | Method for detecting the specificity of activated lymphocyte | HU JUN | 8-
Dec- 03 |
7-
Dec -04 |
9-
Nov -10 |
| US7820210 | Methods and apparatus to prevent, treat, and cure the symptoms of nausea caused by chemotherapy treatments of human cancers | Inhalation, Inc. | 3-
Apr- 00 |
18-
Mar -08 |
26-
Oct- 10 |
| US7812135 | GITR-binding antibodies | TOLERRX, Inc. | 25-
Mar- 05 |
27-
Mar -06 |
12-
Oct- 10 |
| US7803918 | Coronavirus, nucleic acid, protein, and methods for the generation of vaccine, medicaments and diagnostics | Amsterdam Institute of Viral Genomics B.V. | 18-
Aug- 03 |
18-
Aug -04 |
28-
Sep -10 |
| US7803796 | Homopiperazine compounds that inhibit ribosomal frameshifting by binding to RNA pseudoknot structure of SARS coronavirus | Sungkyunkwan University Foundation For Corporate Collaboration | 22-
Dec- 06 |
20-
Dec -07 |
28-
Sep -10 |
| US7803765 | Methods of constructing biodiverse gene fragment libraries and biological modulators isolated therefrom | Phylogica Limited | 20-
Feb- 04 |
20-
Feb -04 |
28-
Sep -10 |
| US7799800 | Lipid-modified immune response modifiers | 3M Innovative Properties Company | 30-
Oct- 03 |
12-
Aug -04 |
21-
Sep -10 |
| US7794998 | Primate T-lymphotropic viruses | Johns Hopkins University | 21-
Feb- 05 |
24-
Feb -07 |
14-
Sep -10 |
| US7794659 | Signal measuring system having a movable signal measuring device | Gen-Probe Incorporated | 10-
Mar- 05 |
10-
Mar -06 |
14-
Sep -10 |
| US7790878 | RNAi modulation of RSV, PIV and other respiratory viruses and uses thereof | Alnylam Pharmaceuticals, Inc. | 22-
Oct- 04 |
25-
Jun -09 |
7-
Sep -10 |
| US7790449 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing the same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
6-
Sep -07 |
7-
Sep -10 |
| US7790159 | Methods, combinations and kits for treating viral infections using immunoconjugates and antibodies to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
7-
Sep -10 |
| US7786290 | Double-stranded ribonucleic acid with increased effectiveness in an organism | Alnylam Pharmaceuticals, Inc. | 13-
Jun- 03 |
14-
Jun -04 |
31-
Aug -10 |
| US7785775 | Human virus causing severe acute respiratory syndrome (SARS) and uses thereof | Versitech Limited | 24-
Mar- 03 |
8-
Jun -07 |
31-
Aug -10 |
| US7785612 | Polyamino acid for use as adjuvant | Masanori Baba | 20-
Apr- 05 |
19-
Apr -06 |
31-
Aug -10 |
| US7781226 | Particle on membrane assay system | The Board of Regents of the University of Texas System | 27-
Feb- 04 |
22-
Dec -04 |
24-
Aug -10 |
| US7781203 | Supports for assaying analytes and methods of making and using thereof | Corning Incorporated | 29-
Dec- 05 |
7-
Jun -06 |
24-
Aug -10 |
| US7777036 | Heterocyclic derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 20-
Sep- 04 |
20-
Sep -05 |
17-
Aug -10 |
| US7777022 | Bioinformatically detectable group of novel regulatory viral and viral associated oligonucleotides and uses thereof | Rosetta Genomics, Ltd. | 26-
Nov- 02 |
26-
May -04 |
17-
Aug -10 |
| US7776521 | Coronavirus isolated from humans | The United States of America as represented by the Secretary of the Department of Health and Human Services, Centers for Disease Control and Prevention | 25-
Apr- 03 |
14-
May -07 |
17-
Aug -10 |
| US7767817 | Water soluble boronic acid fluorescent reporter compounds and methods of use thereof | FANG HAO | 5-
Sep- 03 |
7-
Sep -04 |
3-
Aug -10 |
| US7767677 | Heterocyclic derivatives and their use as stearoyl-CoA desaturase inhibitors | Xenon Pharmaceuticals Inc. | 20-
Sep- 04 |
20-
Sep -05 |
3-
Aug -10 |
| US7767658 | Vaccine composition | Aventis Pasteur SA | 17-
Nov- 03 |
9-
Jan -08 |
3-
Aug -10 |
| US7767657 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
16-
Aug -06 |
3-
Aug -10 |
| US7767210 | RNA virus vaccines and methods | The Board of Regents of the University of Oklahoma | 14-
Dec- 05 |
14-
Dec -06 |
3-
Aug -10 |
| US7763618 | Pyridyl derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 29-
Jul-03 |
29-
Jul- 04 |
27-
Jul- 10 |
| US7758868 | Modified polymerases and attenuated viruses and methods of use thereof | The Penn State Research Foundation | 22-
Dec- 06 |
24-
Dec -07 |
20-
Jul- 10 |
| US7754711 | Pyridazine derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 30-
Jul-03 |
9-
Feb -05 |
13-
Jul- 10 |
| US7750123 | Antibodies against SARS-CoV and methods of use thereof | Dana Farber Cancer Institute, Inc. | 25-
Nov- 03 |
24-
Nov -04 |
6-
Jul- 10 |
| US7749445 | Method and apparatus for analyzing bioprocess fluids | BioScale, Inc. | 2-
May- 05 |
19-
Dec -06 |
6-
Jul- 10 |
| US7745486 | Quercetin-containing compositions | Quercegen Pharma LLC | 17-
Jul-06 |
16-
Jul- 07 |
29-
Jun- 10 |
| US7745442 | Methods of reducing risk of infection from pathogens | Parion Sciences, Inc. | 20-
Aug- 03 |
18-
Aug -04 |
29-
Jun- 10 |
| US7745147 | Methods and uses of antibodies in the purification of interferon | ViraNative AB | 12-
Feb- 05 |
13-
Feb -06 |
29-
Jun- 10 |
| US7745119 | System for detecting polynucleotides | Investigen, Inc. | 20-
May- 03 |
21-
Nov -05 |
29-
Jun- 10 |
| US7745118 | Comparative genomic resequencing | Roche Nimblegen, Inc. | 8-
Apr- 04 |
8-
Apr -05 |
29-
Jun- 10 |
| US7741450 | Antibodies to GM-CSF | Morphotek Inc. | 8-
Feb- 06 |
8-
Feb -07 |
22-
Jun- 10 |
| US7741360 | Bi-aryl or aryl-heteroaryl substituted indoles | AstraZeneca AB | 26-
May- 06 |
25-
May -07 |
22-
Jun- 10 |
| US7740858 | SARS-CoV-specific B-cell epitope and applications thereof | National Taiwan University | 21-
Sep- 04 |
21-
Sep -04 |
22-
Jun- 10 |
| US7737135 | Biphenyloxyacetic acid derivatives for the treatment of respiratory disease | AstraZeneca AB | 24-
Aug- 04 |
22-
Aug -05 |
15-
Jun- 10 |
| US7736850 | Strain of SARS-associated coronavirus and applications thereof | Centre National de la Recherche Scientifique | 2-
Dec- 03 |
2-
Dec -04 |
15-
Jun- 10 |
| US7732177 | Oligoadenylate Synthetase (OAS) | Illumigen Biosciences, Inc. | 23-
Nov- 05 |
17-
Nov -06 |
8-
Jun- 10 |
| US7731978 | Mutant forms of streptolysin O | Novartis AG | 21-
Dec- 07 |
19-
Dec -08 |
8-
Jun- 10 |
| US7728110 | Antibodies to SARS coronavirus | Amgen, Inc. | 19-
May- 06 |
21-
May -07 |
1-
Jun- 10 |
| US7725565 | System and method for detecting, collecting, analyzing, and communicating event related information | Georgetown University | 25-
Feb- 08 |
19-
Nov -08 |
25-
May -10 |
| US7723570 | Edible vaccines expressed in soybeans | SoyMeds, Inc. | 12-
Oct- 04 |
12-
Oct -05 |
25-
May -10 |
| US7723041 | Assay for SARS coronavirus by amplification and detection of the replicase sequence | Becton, Dickinson and Company | 12-
Sep- 03 |
3-
Feb -09 |
25-
May -10 |
| US7722886 | Compositions and methods for treatment of severe acute respiratory syndrome (SARS) | Wyeth | 20-
May- 04 |
20-
May -04 |
25-
May -10 |
| US7714109 | Combinations and kits for cancer treatment using selected antibodies to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
11-
May -10 |
| US7713515 | Methods and compositions for use in diagnosing and characterizing diseases involving abnormal apoptosis | R.E.D. Laboratories N.V. | 27-
May- 03 |
26-
May -04 |
11-
May -10 |
| US7709521 | Substituted indole derivatives for pharmaceutical compositions for treating respiratory diseases | AstraZeneca AB | 18-
Aug- 03 |
16-
Aug -04 |
4-
May -10 |
| US7709511 | Benzothiazolone derivatives | AstraZeneca AB | 9-
Aug- 05 |
3-
Aug -06 |
4-
May -10 |
| US7709188 | Multi-allelic detection of SARS-associated coronavirus | Birch Biomedical Research LLC | 22-
Aug- 03 |
13-
Aug -04 |
4-
May -10 |
| US7700782 | Compounds 569 | AstraZeneca AB | 19-
Dec- 07 |
19-
Dec -07 |
20-
Apr- 10 |
| US7700728 | Use of chimeric receptors in a screening assay for identifying agonists and antagonists of cell receptors | Schering Corporation | 24-
Mar- 05 |
24-
Mar -06 |
20-
Apr- 10 |
| US7700727 | Compositions and kits for detecting pathogen infection | Biokit, S.A. | 23-
Dec- 03 |
27-
Apr -05 |
20-
Apr- 10 |
| US7700273 | Peptidomimetics that mimic a conformational-dependent neutralizing epitope of the human immunodeficiency virus (HIV) CCR5 coreceptor | The United States of America as represented by the Department of Health and Human Services | 9-
Apr- 04 |
11-
Apr -05 |
20-
Apr- 10 |
| US7700120 | Adjuvancy and immune potentiating properties of natural products of Onchocerca volvulus | New York Blood Center | 15-
Jun- 04 |
15-
Jun -05 |
20-
Apr- 10 |
| US7696406 | Expression of a recombinant transgene | Board of Trustees Operating Michigan State University | 3-Jul-
03 |
2-
Jul- 04 |
13-
Apr- 10 |
| US7696330 | Binding molecules against SARS-coronavirus and uses thereof | Crucell Holland B.V. | 22-
Jul-03 |
20-
Jan -06 |
13-
Apr- 10 |
| US7691877 | Pharmaceuticals | Pfizer Inc. | 17-
Feb- 06 |
16-
Feb -07 |
6-
Apr- 10 |
| US7691646 | Hazardous substance removing method, hazardous substance removing material used therein such as air filter, mask, wipe sheet, and the like, and storage method thereof | Daikin Industries, Ltd. | 28-
Mar- 03 |
21-
Nov -08 |
6-
Apr- 10 |
| US7691599 | Mammalian genes involved in viral infection and tumor suppression | Zirus, Inc. | 2-
May- 02 |
2-
May -03 |
6-
Apr- 10 |
| US7691390 | Viral protein | CHANG MING-FU | 2-
Mar- 04 |
19-
Sep -07 |
6-
Apr- 10 |
| US7687535 | Substituted 3-sulfur indoles | AstraZeneca AB | 27-
May- 03 |
25-
May -04 |
30-
Mar -10 |
| US7687475 | RNA interference in respiratory epithelial cells | University of Iowa Research Foundation | 9-Jul-
04 |
16-
Oct -07 |
30-
Mar -10 |
| US7682688 | Microporous materials, methods, and articles for localizing and quantifying analytes | University of Utah Research Foundation | 26-
Nov- 02 |
20-
Nov -03 |
23-
Mar -10 |
| US7678774 | Treating severe acute respiratory syndrome | Hemispherx Biopharma | 16-
May- 03 |
26-
Jan -07 |
16-
Mar -10 |
| US7678386 | Liposomes coated with selected antibodies that bind to aminophospholipids | Board of Regents the University of Texas | 15-
Jul-02 |
15-
Aug -03 |
16-
Mar -10 |
| US7674795 | Fluorene derivatives, composition containing said derivatives and the use thereof | Aventis Pharma SA | 19-
May- 05 |
14-
Nov -07 |
9-
Mar -10 |
| US7670807 | RNA-dependent DNA polymerase from Geobacillus stea rothermophilus | East Tennessee State Univ. Research Foundation | 10-
Mar- 04 |
10-
Mar -04 |
2-
Mar -10 |
| US7670565 | Building decontamination with vaporous hydrogen peroxide | Steris Inc | 31-
Jan- 03 |
3-
Mar -08 |
2-
Mar -10 |
| US7666996 | Casein derived peptides and uses thereof | Peptera Pharmaceuticals Ltd | 1-
Mar- 00 |
1-
Mar -04 |
23-
Feb -10 |
| US7666592 | Methods for concurrent identification and quantification of an unknown bioagent | Ibis Biosciences, Inc. | 18-
Feb- 04 |
17-
Feb -05 |
23-
Feb -10 |
| US7662860 | 3D-structure model of SARS coronavirus 3CL protease and anti- SARS drugs | Shanghai Institute of Materia Medica, Chinese Academy of Sciences | 4-
Jun- 03 |
2-
Dec -05 |
16-
Feb -10 |
| US7648997 | Hydroxylamine substituted imidazoquinolines | Coley Pharmaceutical Group, Inc. | 12-
Aug- 03 |
12-
Aug -04 |
19-
Jan- 10 |
| US7648844 | Method and apparatus for detection of analyte using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
19-
Jan- 10 |
| US7645881 | Methods for treating hepatitis C | PTC Therapeutics, Inc. | 22-
Jul-04 |
14-
Jul- 05 |
12-
Jan- 10 |
| US7642350 | Purine derivatives | Pfizer Limited | 4-
May- 05 |
3-
May -06 |
5-
Jan- 10 |
| US7636637 | Variable length probe selection | Roche NimbleGen, Inc. | 18-
Jun- 04 |
20-
Jun -05 |
22-
Dec -09 |
| US7635557 | Enzymatic diagnostic test for SARS and other viral diseases | MND Diagnostic Ltd. | 23-
Jun- 03 |
23-
Jun -04 |
22-
Dec -09 |
| US7635485 | Method of accelerated vaccination against Ebola viruses | The United States of America as represented by the Department of Health and Human Services | 1-
Aug- 03 |
17-
Jan -06 |
22-
Dec -09 |
| US7632638 | Methods and apparatus for detecting viruses using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
15-
Dec -09 |
| US7629443 | Neutralizing monoclonal antibodies against severe acute respiratory syndrome-associated coronavirus | New York Blood Center, Inc. | 2-
Jun- 04 |
8-
Feb -06 |
8-
Dec -09 |
| US7629385 | Sphingolipid-derived pharmaceutical compositions | Jado Technologies GmbH | 29-
Jun- 04 |
29-
Jun -05 |
8-
Dec -09 |
| US7629137 | Methods and apparatus for detecting bacteria using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
8-
Dec -09 |
| US7629114 | Method of collecting nasopharyngeal cells and secretions for diagnosis of viral upper respiratory infections and screening for nasopharyngeal cancer | World Sense Technology Limited | 26-
Aug- 03 |
20-
Aug -04 |
8-
Dec -09 |
| US7625563 | Cancer treatment methods using selected immunoconjugates for binding to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
1-
Dec -09 |
| US7625492 | Charge-based water filtration systems | The University of Wyoming Research Corporation | 28-
Jan- 03 |
28-
Jan -04 |
1-
Dec -09 |
| US7625428 | Bioagent air filtration systems | The University of Wyoming Research Corporation | 28-
Jan- 03 |
28-
Jan -04 |
1-
Dec -09 |
| US7623997 | Computer-implemented biological sequence identifier system and method | The United States of America as represented by the Secretary of the Navy | 2-Jul-
04 |
6-
Jun -06 |
24-
Nov -09 |
| US7622559 | Human monoclonal antibodies against interleukin 8 (IL-8) | Genmab A/S | 16-
Dec- 02 |
27-
Jun -07 |
24-
Nov -09 |
| US7622125 | Polycistronic HIV vector constructs | Novartis Vaccines and Diagnostics, Inc. | 5-
May- 04 |
5-
May -05 |
24-
Nov -09 |
| US7622118 | Cancer treatment methods using selected antibodies to a minophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
24-
Nov -09 |
| US7622112 | Anti-SARS monoclonal antibodies | Not Available | 5-
Dec- 03 |
6-
Dec -04 |
24-
Nov -09 |
| US7619067 | Evolved interferon-alpha polypeptides | Maxygen, Inc. | 18-
May- 05 |
17-
May -06 |
17-
Nov -09 |
| US7618802 | Compositions of coronaviruses with a recombination-resistant genome | The University of North Carolina at Chapel Hill | 21-
Jul-03 |
19-
Jan -06 |
17-
Nov -09 |
| US7618788 | Proteome epitope tags and methods of use thereof in protein modification analysis | Millipore Corporation | 10-
May- 02 |
5-
Feb -04 |
17-
Nov -09 |
| US7618635 | Super-antigen fusion proteins and the use thereof | Healthbanks Biotech Co., Ltd. | 21-
Jul-04 |
19-
Jul- 05 |
17-
Nov -09 |
| US7615381 | Method and apparatus for detecting estradiol and metabolites thereof using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
10-
Nov -09 |
| US7615223 | Selected immunoconjugates for binding to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
10-
Nov -09 |
| US7611908 | Method and apparatus for therapeutic drug monitoring using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
3-
Nov -09 |
| US7611704 | Compositions and methods for treating viral infections using antibodies and immunoconjugates to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
3-
Nov -09 |
| US7605161 | Pyridyl derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 30-
Jul-03 |
29-
Jul- 04 |
20-
Oct- 09 |
| US7605135 | Baicalin as a treatment for SARS infection | The University of Hong Kong | 10-
Nov- 03 |
8-
Nov -04 |
20-
Oct- 09 |
| US7604960 | Transient protein expression methods | Crucell Holland B.V. | 15-
Apr- 99 |
1-
Jun -07 |
20-
Oct- 09 |
| US7604801 | Methods for detecting parvovirus infections | The Research Foundation of State University of New York | 5-
May- 03 |
16-
Oct -08 |
20-
Oct- 09 |
| US7598382 | Aryl substituted imidazoquinolines | Coley Pharmaceutical Group, Inc. | 20-
Dec- 02 |
13-
Jan -06 |
6-
Oct- 09 |
| US7598094 | Methods and apparatus for detecting cardiac injury markers using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
6-
Oct- 09 |
| US7598072 | Assay to detect viral uncoating | Wisconsin Alumni Research Foundation | 9-
Dec- 03 |
8-
Dec -04 |
6-
Oct- 09 |
| US7597936 | Method of producing a pigmented composite microporous material | University of Utah Research Foundation | 26-
Nov- 02 |
26-
May -04 |
6-
Oct- 09 |
| US7595381 | Method for detecting SARS coronavirus | Eiken Kagaku Kabushiki Kaisha | 27-
Jun- 03 |
10-
Jun -08 |
29-
Sep -09 |
| US7595163 | Method for detecting SARS coronavirus | Eiken Kagaku Kabushiki Kaisha | 27-
Jun- 03 |
10-
Jun -08 |
29-
Sep -09 |
| US7592343 | Pyridazine-piperazine compounds and their use as stearoyl-CoA desaturase inhibitors | Xenon Pharmaceuticals Inc. | 20-
Sep- 04 |
20-
Sep -05 |
22-
Sep -09 |
| US7592322 | RNAi modulation of RSV, PIV and other respiratory viruses and uses thereof | Alnylam Pharmaceuticals, Inc. | 22-
Oct- 04 |
14-
Jun -05 |
22-
Sep -09 |
| US7592008 | Membrane scaffold proteins | The Board of Trustees of the University of Illinois, a body corporate and politic of the State of Illinois | 20-
Nov- 00 |
11-
Jan -05 |
22-
Sep -09 |
| US7589092 | Prodrugs of heteroaryl compounds | Koronis Pharmaceuticals, Incorporated | 20-
Jun- 03 |
27-
Dec -06 |
15-
Sep -09 |
| US7585647 | Nucleic acid encoding recombinant interferon | WEI GUANGWEN | 28-
Aug- 03 |
26-
Aug -04 |
8-
Sep -09 |
| US7582740 | Methods and kits for detecting SARS-associated coronavirus | The Trustees of Columbia University In the City of New York | 17-
Apr- 03 |
23-
Jan -04 |
1-
Sep -09 |
| US7582621 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
16-
Feb -06 |
1-
Sep -09 |
| US7579396 | Polymer composite | Eastman Kodak Company | 31-
Jan- 07 |
31-
Jan -07 |
25-
Aug -09 |
| US7579359 | 1-alkoxy lH-imidazo ring systems and methods | 3M Innovative Properties Company | 2-
Sep- 04 |
1-
Sep -05 |
25-
Aug -09 |
| US7572621 | Detection, characterization and treatment of viral infection and methods thereof | Canadian Blood Services | 9-
Apr- 03 |
11-
Oct -05 |
11-
Aug -09 |
| US7572448 | Combined cancer treatment methods using selected antibodies to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
11-
Aug -09 |
| US7572442 | Selected antibody compositions for binding to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Jul- 03 |
11-
Aug -09 |
| US7569536 | Method for controlling SR protein phosphorylation, and antiviral agents whose active ingredients comprise agents that control SR protein activity | Masatoshi Hagiwara | 26-
Dec- 03 |
24-
Dec -04 |
4-
Aug -09 |
| US7569384 | Albumin fusion proteins | Human Genome Sciences, Inc. | 9-
Feb- 04 |
8-
Aug -06 |
4-
Aug -09 |
| US7550140 | Antibody to the human OX40 receptor | Crucell Holland B.V. | 13-
Jun- 02 |
13-
Jun -03 |
23-
Jun- 09 |
| US7547698 | Bicyclic heterocyclic derivatives and their use as inhibitors of stearoyl-coadesaturase (SCD) | Xenon Pharmaceuticals Inc. | 20-
Sep- 04 |
20-
Sep -05 |
16-
Jun- 09 |
| US7547516 | Method for reducing the presence of amplification inhibitors in a reaction receptacle | Gen-Probe Incorporated | 10-
Mar- 05 |
10-
Mar -06 |
16-
Jun- 09 |
| US7547512 | High-throughput diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS) | The University of Hong Kong | 24-
Mar- 03 |
24-
Mar -04 |
16-
Jun- 09 |
| US7544697 | Pyrazolopyridines and analogs thereof | Coley Pharmaceutical Group, Inc. | 3-
Oct- 03 |
1-
Apr -05 |
9-
Jun- 09 |
| US7541436 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 19-
May- 04 |
4-
May -07 |
2-
Jun- 09 |
| US7541163 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 19-
May- 04 |
14-
Aug -07 |
2-
Jun- 09 |
| US7531630 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 19-
May- 04 |
13-
Sep -06 |
12-
May -09 |
| US7531324 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 19-
May- 04 |
13-
Sep -06 |
12-
May -09 |
| US7521424 | Albumin fusion proteins | Human Genome Sciences, Inc. | 22-
Jan- 03 |
7-
Jul- 05 |
21-
Apr- 09 |
| US7521185 | Assay for SARS coronavirus by amplification and detection of the replicase sequence | Becton, Dickinson and Company | 12-
Sep- 03 |
13-
Sep -04 |
21-
Apr- 09 |
| US7514436 | Pyridazine derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 30-
Jul-03 |
29-
Jul- 04 |
7-
Apr- 09 |
| US7511124 | Compositions comprising phosphatidylethanolamine-binding peptides linked to anti-viral agents | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
31-
Mar -09 |
| US7504384 | Use of lipid conjugates in the treatment of infection | Yissum Research Development Company of the Hebrew University of Jerusalem | 10-
Jan- 00 |
8-
Sep -05 |
17-
Mar -09 |
| US7504382 | Protease inhibitors for coronaviruses and SARS-CoV and the use thereof | Cytovia, Inc. | 6-
May- 03 |
6-
May -04 |
17-
Mar -09 |
| US7504205 | Uncharacterized ORF3 in SARS-coronavirus is a cyclic-AMP- dependent kinase and a target for SARS therapy | The Burnham Institute | 17-
May- 04 |
17-
May -05 |
17-
Mar -09 |
| US7504097 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 18-
Nov- 02 |
30-
Oct -06 |
17-
Mar -09 |
| US7498409 | Screening assay for TLR7, TLR8 and TLR9 agonists and antagonists | Schering Corporation | 24-
Mar- 05 |
23-
Mar -06 |
3-
Mar -09 |
| US7498152 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 18-
Nov- 02 |
30-
Oct -06 |
3-
Mar -09 |
| US7495011 | Anti-coronavirus drug | aRigen Pharmaceuticals, Inc. | 15-
Jul-03 |
14-
Jul- 04 |
24-
Feb -09 |
| US7491793 | Influenza virus inhibiting peptides | The Administrators of the Tulane Educational Fund | 4-
Nov- 03 |
3-
Nov -04 |
17-
Feb -09 |
| US7491706 | Artificial cpg single-stranded oligodeoxynucleotide and antiviral use thereof | Changchun Huapu Biotechnology Co., Ltd. | 25-
Jul-03 |
26-
Jul- 04 |
17-
Feb -09 |
| US7491508 | Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses | The Trustees of the University of Pennsylvania | 20-
Jun- 03 |
15-
Jun -04 |
17-
Feb -09 |
| US7491489 | Synthetic peptide targeting critical sites on the SARS-associated coronavirus spike protein responsible for viral infection and method of use thereof | The University of Hong Knog | 22-
Nov- 04 |
28-
Oct -05 |
17-
Feb -09 |
| US7491397 | Receptor binding polypeptides | National Health Research Institutes | 9-
Jan- 04 |
10-
Jan -05 |
17-
Feb -09 |
| US7488801 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 18-
Nov- 02 |
30-
Oct -06 |
10-
Feb -09 |
| US7488589 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 18-
Nov- 02 |
30-
Oct -06 |
10-
Feb -09 |
| US7488473 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 18-
Nov- 02 |
30-
Oct -06 |
10-
Feb -09 |
| US7485432 | Selective modulation of TLR-mediated biological activity | 3M Innovative Properties Company | 27-
Feb- 03 |
27-
Feb -04 |
3-
Feb -09 |
| US7482334 | Therapeutic treatment methods | Hollis-Eden Pharmaceuticals, Inc. | 28-
Aug- 02 |
12-
Feb -07 |
27-
Jan- 09 |
| US7482149 | Inhibition of SARS coronavirus infection with clinically approved antiviral drugs | Genome Institute of Singapore | 9-
Jun- 03 |
9-
Jun -04 |
27-
Jan- 09 |
| US7479484 | Peptides and peptidomimetics having immune-modulating, antiinflammatory, and anti-viral activity | Takeda Pharmaceutical Company Limited | 25-
Jun- 03 |
25-
Jun -04 |
20-
Jan- 09 |
| US7470666 | Use of Ulinastatin and its pharmaceutical composition for treating severe acute respiratory syndrome | Guangdong Techpool Biochem. Pharma. Co., Ltd. | 26-
May- 03 |
25-
May -04 |
30-
Dec -08 |
| US7470548 | Hazardous substance removing method, hazardous substance removing material used therein such as air filter, mask, wipe sheet, and the like, and storage method thereof | Daikin Industries, Ltd. | 28-
Mar- 03 |
26-
Mar -04 |
30-
Dec -08 |
| US7468418 | Compositions for enhancing transport of molecules into cells | AVI BioPharma., Inc. | 29-
Apr- 03 |
29-
Apr -04 |
23-
Dec -08 |
| US7465836 | Hydrolytically-resistant boron-containing therapeutics and methods of use | Anacor Pharmaceuticals, Inc. | 16-
Jun- 03 |
15-
Jun -04 |
16-
Dec -08 |
| US7462615 | Inhibitors of cysteine proteases, the pharmaceutical compositions thereof and their therapeutic applications | Hybrigenics SA | 8-
Dec- 05 |
8-
Dec -05 |
9-
Dec -08 |
| US7460960 | Proteome epitope tags and methods of use thereof in protein modification analysis | Epitome Biosystems, Inc. | 10-
May- 02 |
13-
Nov -03 |
2-
Dec -08 |
| US7456180 | Piperazine derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 30-
Jul-03 |
29-
Jul- 04 |
25-
Nov -08 |
| US7455833 | Methods and compositions for treating viral infections using antibodies and immunoconjugates to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
25-
Nov -08 |
| US7452542 | Live attenuated coronavirus vaccines | Vanderbilt University | 21-
May- 04 |
23-
May -05 |
18-
Nov -08 |
| US7445889 | Methods for detecting parvovirus infections | The Research Foundation of State University of New York | 5-
May- 03 |
5-
May -04 |
4-
Nov -08 |
| US7442761 | Replikin peptides and uses thereof | BOGOCH ELENORE S | 6-
Jun- 03 |
4-
Jun -04 |
28-
Oct- 08 |
| US7442508 | Methods for detection and production of influenza viruses | Diagnostic Hybrids, Inc. | 24-
Apr- 98 |
28-
Apr -06 |
28-
Oct- 08 |
| US7439349 | Method for preparation of large volume batches of poly-ICLC with increased biological potency; therapeutic, clinical and veterinary uses thereof | Not Available | 3-Jul-
02 |
1-
Jul- 03 |
21-
Oct- 08 |
| US7439052 | Method of making modified immunodeficiency virus particles | Lipid Sciences | 29-
Jun- 00 |
10-
Apr -06 |
21-
Oct- 08 |
| US7435588 | Systems for detection and production of respiratory, herpes and enteric viruses | Diagnostic Hybrids, Inc. | 20-
Sep- 05 |
28-
Apr -06 |
14-
Oct- 08 |
| US7435538 | High throughput screening method of drug for physiologically active protein | CellFree Sciences Co., Ltd. | 8-
Sep- 03 |
8-
Sep -04 |
14-
Oct- 08 |
| US7432045 | Method of inhibiting influenza infection with antiviral peptides | Wisconsin Alumni Research Foundation | 1-
Dec- 03 |
1-
Dec -04 |
7-
Oct- 08 |
| US7429656 | Inhibition of SARS-associated coronavirus (SCoV) infection and replication by RNA interference | The University of Hong Kong | 19-
May- 03 |
14-
Jun -06 |
30-
Sep -08 |
| US7427479 | Methods and kits for identifying target nucleotides in mixed populations | Applera Corporation | 30-
Apr- 04 |
29-
Apr -05 |
23-
Sep -08 |
| US7424370 | Computational method for identifying adhesin and adhesin-like proteins of therapeutic potential | Council of Scientific and Industrial Research | 20-
Jul-04 |
7-
Feb -05 |
9-
Sep -08 |
| US7407663 | Modified immunodeficiency virus particles | Lipid Sciences, Inc. | 29-
Jun- 00 |
20-
Jun -03 |
5-
Aug -08 |
| US7407662 | Modified viral particles with immunogenic properties and reduced lipid content | Lipid Sciences, Inc. | 29-
Jun- 00 |
21-
Jun -04 |
5-
Aug -08 |
| US7405207 | Nebulizer formulations of dehydroepiandrosterone and methods of treating asthma or chronic obstructive pulmonary disease using compositions thereof | Epigenesis Pharmaceuticals, Inc. | 17-
Jun- 02 |
17-
Jun -03 |
29-
Jul- 08 |
| US7405046 | Compositions and methods for treatment of rhinovirus | The Quigley Corporation | 6-
Aug- 01 |
27-
Oct -06 |
29-
Jul- 08 |
| US7399588 | Method for detecting SARS coronavirus | Eiken Kagaku Kabushiki Kaisha | 27-
Jun- 03 |
15-
Jun -04 |
15-
Jul- 08 |
| US7396914 | SARS nucleic acids, proteins, antibodies, and uses thereof | University of Massachusetts | 4-
Aug- 03 |
4-
Aug -04 |
8-
Jul- 08 |
| US7393856 | Anti-viral uses of borinic acid complexes | Anacor Pharmaceuticals, Inc. | 14-
Jun- 04 |
14-
Jun -05 |
1-
Jul- 08 |
| US7393638 | Assay system and methods for detecting SARS-CV | AsiaGEN Corporation | 1-Jul-
03 |
1-
Jul- 03 |
1-
Jul- 08 |
| US7387271 | Immunostimulatory combinations | 3M Innovative Properties Company | 30-
Dec- 02 |
30-
Dec -03 |
17-
Jun- 08 |
| US7384909 | Anti-viral treatment methods using phosphatidylethanolaminebinding peptides linked to anti-viral agents | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
10-
Jun- 08 |
| US7378386 | Anti-viral treatment methods using phosphatidylethanolaminebinding peptide derivatives | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
27-
May -08 |
| US7375210 | PCR primer set for detecting severe acute respiratory syndrome (SARS)-Coronavirus, method and kit for detecting SARS- Coronavirus using the same | Samsung Electronics Co., Ltd. | 12-
Dec- 03 |
24-
Nov -04 |
20-
May -08 |
| US7375202 | Human virus causing severe acute respiratory syndrome (SARS) and uses thereof | The University of Hong Kong | 24-
Mar- 03 |
24-
Mar -04 |
20-
May -08 |
| US7375180 | Methods and compositions related to IRM compounds and Toll-like receptor 8 | 3M Innovative Properties Company | 13-
Feb- 03 |
12-
Feb -04 |
20-
May -08 |
| US7374883 | Method and kit for the detection of a novel coronoavirus associated with the severe acute respiratory syndrome (SARS) | QIAGEN Diagnostics GmbH | 30-
Apr- 03 |
30-
Apr -04 |
20-
May -08 |
| US7371850 | Method and composition for reducing expression of ROCK-II | Myriad Genetics, Inc. | 20-
Aug- 03 |
20-
Aug -04 |
13-
May -08 |
| US7371837 | Human virus causing respiratory tract infection and uses thereof | The University of Hong Kong | 21-
Jul-04 |
16-
May -05 |
13-
May -08 |
| US7371525 | Compositions and methods for diagnosing and treating severe acute respiratory syndrome (SARS) | The Chinese University of Hong Kong | 29-
Jul-03 |
28-
Jul- 04 |
13-
May -08 |
| US7361747 | Isolation and characterization of the precursor virus of human SARS virus: SARS-associated corona virus-like virus | The University of Hong Kong | 22-
May- 03 |
24-
May -04 |
22-
Apr- 08 |
| US7361304 | Building decontamination with vaporous hydrogen peroxide | Steris Inc. | 31-
Jan- 03 |
29-
Jan -04 |
22-
Apr- 08 |
| US7358068 | Antiviral oligonucleotides | Replicor, Inc. | 13-
Sep- 02 |
12-
Sep -03 |
15-
Apr- 08 |
| US7354908 | Materials and methods for prevention and treatment of RNA viral diseases | University of South Florida | 30-
Apr- 02 |
30-
Apr -03 |
8-
Apr- 08 |
| US7354551 | Room decontamination with hydrogen peroxide vapor | Steris Inc | 8-Jul-
04 |
8-
Jul- 04 |
8-
Apr- 08 |
| US7344740 | Methods and apparatus to prevent, treat, and cure the symptoms of nausea caused by chemotherapy treatments of human cancers | Inhalation, Inc. | 3-
Apr- 00 |
27-
Nov -04 |
18-
Mar -08 |
| US7344720 | Vaccine composition | Sanofi Pasteur SA | 17-
Nov- 03 |
15-
Nov -04 |
18-
Mar -08 |
| US7339051 | Compositions and methods for the treatment of severe acute respiratory syndrome (SARS) | Isis Pharmaceuticals, Inc. | 28-
Apr- 03 |
26-
Apr -04 |
4-
Mar -08 |
| US7335658 | Pyridazine derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 30-
Jul-03 |
29-
Jul- 04 |
26-
Feb -08 |
| US7332475 | Preventive or therapeutic composition for viral infectious disease | Kyowa Hakko Kogyo Co., Ltd. | 22-
Jul-03 |
22-
Jul- 04 |
19-
Feb -08 |
| US7332294 | CXCL10-based diagnosis and treatment of respiratory illnesses | University Health Network | 17-
Aug- 04 |
17-
Aug -04 |
19-
Feb -08 |
| US7320857 | Characterization of the earliest stages of the severe acute respiratory syndrome (SARS) virus and uses thereof | Chinese National Human Genome Center at Shanghai | 9-Jul-
04 |
9-
Jul- 04 |
22-
Jan- 08 |
| US7318918 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 19-
May- 04 |
18-
May -05 |
15-
Jan- 08 |
| US7314613 | Interferon-alpha polypeptides and conjugates | Maxygen, Inc. | 18-
Nov- 02 |
19-
May -04 |
1-
Jan- 08 |
| US7312036 | Compositions for use in identification of viral hemorrhagic fever viruses | ISIS Pharmaceuticals, Inc. | 22-
Mar- 04 |
21-
Mar -05 |
25-
Dec -07 |
| US7297786 | RNA interference in respiratory epitheial cells | University of Iowa Research Foundation | 9-Jul-
04 |
11-
Jul- 05 |
20-
Nov -07 |
| US7291498 | Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses | The Trustees of the University of Pennsylvania | 20-
Jun- 03 |
20-
Jun -03 |
6-
Nov -07 |
| US7282568 | Human monoclonal antibodies against interleukin 8 (IL-8) | Genmab A/S | 16-
Dec- 02 |
16-
Dec -03 |
16-
Oct- 07 |
| US7282199 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
25-
Apr -03 |
16-
Oct- 07 |
| US7267942 | Diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS) | The University of Hong Kong | 24-
Mar- 03 |
24-
Mar -04 |
11-
Sep -07 |
| US7261867 | Production of silver sulfate grains using organo-sulfate or organo- sulfonate additives | Eastman Kodak Company | 7-
Apr- 06 |
7-
Apr -06 |
28-
Aug -07 |
| US7247303 | Selected antibody CDRs for binding to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
15-
Aug -03 |
24-
Jul- 07 |
| US7244732 | Prodrugs of heteroaryl compounds | Koronis Pharmaceuticals, Incorporated | 20-
Jun- 03 |
31-
Mar -04 |
17-
Jul- 07 |
| US7223787 | Prenylation inhibitors reduce host cell permissiveness to viral replication | Board of Regents, The University of Texas System | 21-
Oct- 03 |
21-
Oct -03 |
29-
May -07 |
| US7220852 | Coronavirus isolated from humans | The United States of America as represented by the Secretary of the Department of Health and Human Services, Centers for Disease Control and Prevention | 25-
Apr- 03 |
12-
Apr -04 |
22-
May -07 |
| US7183300 | Inhibitors of HIV-1 capsid formation: substituted aryl aminomethyl thiazole ureas and analogues thereof | AGARWAL ATUL | 11-
Nov- 04 |
10-
Nov -05 |
27-
Feb -07 |
| US7166435 | Compositions and methods for reducing the transmissivity of illnesses | The Quigley Corporation | 6-
Aug- 01 |
14-
Dec -04 |
23-
Jan- 07 |
| US7163947 | 1-Amino lH-imidazoquinolines | 3M Innovative Properties Company | 7-
Mar- 03 |
3-
Sep -04 |
16-
Jan- 07 |
| US7151163 | Antiviral agents for the treatment, control and prevention of infections by coronaviruses | Sequoia Pharmaceuticals, Inc. | 28-
Apr- 03 |
28-
Apr -04 |
19-
Dec -06 |
| US7151091 | Compositions and methods for preventing infection | La Jolla Biosciences LLC | 20-
Sep- 02 |
22-
Sep -03 |
19-
Dec -06 |
| US7148248 | Method of treating or inhibiting the development of brain inflammation and sepsis | NOZAKI MASAKO | 29-
Nov- 02 |
26-
Nov -03 |
12-
Dec -06 |
| US7129223 | Inhibition of SARS-associated coronavirus (SCoV) infection and replication by RNA interference | The University of HongKong | 19-
May- 03 |
19-
May -04 |
31-
Oct- 06 |
| US7129042 | Compositions and methods for detecting severe acute respiratory syndrome coronavirus | Diagnostic Hybrids, Inc. | 3-
Nov- 03 |
3-
Nov -03 |
31-
Oct- 06 |
| US7115563 | Composition and its therapeutic use | Insignion Holding Limited | 29-
May- 02 |
29-
May -03 |
3-
Oct- 06 |
| US7091214 | Aryl substituted Imidazoquinolines | 3M Innovative Properties Co. | 20-
Dec- 02 |
18-
Dec -03 |
15-
Aug -06 |
| US7048953 | Methods and apparatus to prevent, treat and cure infections of the human respiratory system by pathogens causing severe acute respiratory syndrome (SARS) | Inhalation, Inc. | 3-
Apr- 00 |
2-
May -03 |
23-
May -06 |
| US7023593 | Apparatus for forming nano-grating device | Industrial Technology Research Institute | 28-
Nov- 03 |
17-
Mar -04 |
4-
Apr- 06 |
| US6946291 | Mixed cell diagnostic systems | Diagnostic Hybrids, Inc. | 24-
Apr- 98 |
30-
Mar -04 |
20-
Sep -05 |
| US20200176
079 |
METHODS AND DEVICES FOR NUCLEIC ACID-BASED REAL-TIME DETERMINATION OF DISEASE STATES | Not Available | 19-
Jul-17 |
18-
Jul- 18 |
4-
Jun- 20 |
| US20200173
925 |
MICROSCOPIC BODY ENCLOSING METHOD, MICROSCOPIC BODY DETECTION METHOD, AND MICROSCOPIC BODY DETECTION DEVICE | JAPAN SCIENCE AND TECHNOLOGY AGENCY | 29-
Mar- 17 |
28-
Mar -18 |
4-
Jun- 20 |
| US20200172
883 |
COMPOSITIONS FOR INCREASING POLYPEPTIDE STABILITY AND ACTIVITY, AND RELATED METHODS | Not Available | 19-
Nov- 09 |
13-
Sep -19 |
4-
Jun- 20 |
| US20200172
879 |
DHFR TUNABLE PROTEIN REGULATION | Not Available | 3-
Mar- 17 |
2-
Sep -19 |
4-
Jun- 20 |
| US20200172
600 |
RSV F PROTEIN COMPOSITIONS AND METHOD FOR MAKING SAME | GLAXOSMITHKLINE BIOLOGICALS, SA | 15-
Jul-09 |
16-
Aug -17 |
4-
Jun- 20 |
| US20200172
513 |
DIHYDROPYRIMIDINYL BENZAZEPINE CARBOXAMIDE COMPOUNDS | Hoffmann-La Roche Inc. | 12-
Jun- 16 |
5-
Feb -20 |
4-
Jun- 20 |
| US20200172
480 |
CONJUGATES OF CELL BINDING MOLECULES WITH CYTOTOXIC AGENTS | HANGZHOU DAC BIOTECH CO., LTD. | 12-
Jul-12 |
5-
Feb -20 |
4-
Jun- 20 |
| US20200171
085 |
Method of Treating Respiratory Tract Infection | Not Available | 19-
May- 17 |
18-
May -18 |
4-
Jun- 20 |
| US20200171
060 |
FLEX-NUCLEOSIDE ANALOGUES, NOVEL THERAPEUTICS AGAINST FILOVIRUSES AND FLAVIVIRUSES | Not Available | 31-
Jul-17 |
26-
Jan -18 |
4-
Jun- 20 |
| US20200166
505 |
METHOD AND DEVICE FOR DETECTING ANTIGEN-SPECIFIC ANTIBODIES IN A BIOLOGICAL FLUID SAMPLE BY USING NEODYMIUM MAGNETS | The U.S.A., as represented by the Secretary, Department of Health and Human Services | 1-
Sep- 15 |
30-
Jan -20 |
28-
May -20 |
| US20200165
632 |
ENHANCING AGENTS FOR IMPROVED CELL TRANSFECTION AND/OR rAAV VECTOR PRODUCTION | Spark Therapeutics, Inc. | 7-
Jun- 17 |
6-
Jun -18 |
28-
May -20 |
| US20200165
630 |
COMPOSITIONS FOR THE TREATMENT OF DISEASE | Not Available | 29-
Apr- 16 |
28-
Apr -17 |
28-
May -20 |
| US20200165
613 |
VIRUS LIKE PARTICLE | The University of Leeds | 1-
Jun- 17 |
31-
May -18 |
28-
May -20 |
| US20200165
594 |
CRISPR SYSTEM BASED ANTIVIRAL THERAPY | MASSACHUSETTS INSTITUTE OF TECHNOLOGY | 7-Jul-
17 |
6-
Jul- 18 |
28-
May -20 |
| US20200165
585 |
NEW CHIMERIC ENZYMES AND THEIR APPLICATIONS | Not Available | 27-
Jul-17 |
27-
Jul- 18 |
28-
May -20 |
| US20200165
357 |
CARBONIC ANHYDRASE IX (G250) ANTIBODIES AND METHODS OF USE THEREOF | Not Available | 2-
Dec- 05 |
21-
Oct -19 |
28-
May -20 |
| US20200164
334 |
MICROSPOTTING DEVICE | Not Available | 18-
Apr- 12 |
11-
Oct -19 |
28-
May -20 |
| US20200164
067 |
Methods and Compositions for Inhibiting Akt3 | Not Available | 15-
Jan- 16 |
5-
Feb -20 |
28-
May -20 |
| US20200164
058 |
TRIMERIC S1-CD40L FUSION PROTEIN VACCINE AGAINST MIDDLE EAST RESPIRATORY SYNDROME-CORONAVIRUS | King Abdulaziz University | 27-
Nov- 18 |
27-
Nov -18 |
28-
May -20 |
| US20200164
020 |
PUM 1 PROTEIN AS TARGET FOR VIRUS INHIBITION | Not Available | 12-
Apr- 17 |
12-
Apr -17 |
28-
May -20 |
| US20200163
878 |
LIPID NANOPARTICLE MRNA VACCINES | Not Available | 26-
Oct- 16 |
26-
Oct -17 |
28-
May -20 |
| US20200157
600 |
METHODS AND COMPOSITIONS FOR WHOLE TRANSCRIPTOME AMPLIFICATION | Not Available | 19-
Nov- 18 |
19-
Nov -18 |
21-
May -20 |
| US20200157
222 |
ANTI-PD-L1 ANTIBODIES AND USES THEREOF | Not Available | 29-
Mar- 18 |
29-
Mar -19 |
21-
May -20 |
| US20200157
221 |
ONCOLYTIC VIRAL DELIVERY OF THERAPEUTIC POLYPEPTIDES | Not Available | 30-
Jun- 16 |
28-
Jan -20 |
21-
May -20 |
| US20200155
704 |
ADENO-ASSOCIATED VIRUS (AAV) CLADES, SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 30-
Sep- 03 |
30-
Jan -20 |
21-
May -20 |
| US20200155
699 |
MODIFIED VIRUS-LIKE PARTICLES OF CMV | Not Available | 22-
Oct- 14 |
26-
Nov -19 |
21-
May -20 |
| US20200155
667 |
VACCINE COMPOSITIONS | Not Available | 27-
Jul-17 |
24-
Jan -20 |
21-
May -20 |
| US20200155
664 |
INFLUENZA VACCINES WITH REDUCED AMOUNTS OF SQUALENE | Not Available | 10-
Feb- 09 |
26-
Jun -19 |
21-
May -20 |
| US20200155
662 |
COMBINATION IMMUNOTHERAPIES COMPRISING IL-15 SUPERAGONISTS | Not Available | 26-
May- 17 |
25-
May -18 |
21-
May -20 |
| US20200155
660 |
COMPOSITIONS AND METHODS FOR MODIFIED DENDRIMER NANOPARTICLE DELIVERY | Not Available | 23-
Sep- 15 |
22-
Nov -19 |
21-
May -20 |
| US20200155
646 |
EV576 For Use in the Treatment of Viral Infections of the Respiratory Tract | Volution Immuno Pharmaceuticals SA | 8-
Jan- 10 |
7-
Jun -19 |
21-
May -20 |
| US20200149
062 |
ENGINEERED TSC2 | Not Available | 14-
Jul-17 |
13-
Jul- 18 |
14-
May -20 |
| US20200149
048 |
Transbiotic Regulation of Bacterial Gene Expression | Not Available | 22-
May- 17 |
22-
May -18 |
14-
May -20 |
| US20200148
749 |
ANTIBODIES AND PROCESSES FOR PREPARING THE SAME | Not Available | 16-
May- 08 |
10-
Oct -19 |
14-
May -20 |
| US20200147
203 |
VACCINATION OF IMMUNOCOMPROMISED SUBJECTS | Not Available | 26-
Sep- 14 |
10-
Jan -20 |
14-
May -20 |
| US20200147
193 |
COMPOSITIONS AND METHODS FOR TUMOR VACCINATION AND IMMUNOTHERAPY INVOLVING HER ANTIGENS | Not Available | 2-
Jun- 17 |
2-
Jun -18 |
14-
May -20 |
| US20200147
171 |
COMPOSITIONS OF CRACC FUSIONS AND METHODS FOR MODULATING AN IMMUNE RESPONSE AGAINST CANCERS, INFECTIONS DISEASES AND DISORDERS | Not Available | 18-
Sep- 18 |
18-
Sep -19 |
14-
May -20 |
| US20200140
547 |
MULTIFUNCTIONAL ANTIBODY-LIGAND TRAPS TO MODULATE IMMUNE TOLERANCE | Not Available | 26-
May- 17 |
25-
May -18 |
7-
May -20 |
| US20200140
493 |
Engineering Virus-like Nanocarriers for Biomolecule Delivery | Not Available | 26-
Oct- 18 |
25-
Oct -19 |
7-
May -20 |
| US20200140
398 |
HUMAN HELICASE DDX3 INHIBITORS AS THERAPEUTIC AGENTS | AZIENDA OSPEDALIERA UNIVERSITARIA SENESE | 13-
Feb- 15 |
6-
Jan -20 |
7-
May -20 |
| US20200138
937 |
Genetically Attenuated Nucleic Acid Vaccine | Not Available | 2-
Jun- 17 |
1-
Jun -18 |
7-
May -20 |
| US20200138
936 |
Phenotypically Wild-Type and Genetically Attenuated Viruses | Not Available | 2-
Jun- 17 |
1-
Jun -18 |
7-
May -20 |
| US20200138
818 |
METHODS OF TREATING PAIN AND/OR INFLAMMATORY DISORDERS USING LAPATINIB | Not Available | 7-
Nov- 18 |
6-
Nov -19 |
7-
May -20 |
| US20200138
780 |
METHODS FOR TREATING PULMONARY EMPHYSEMA USING SUBSTITUTED 2-AZA-BICYCLO[2.2.1]HEPTANE-3-CARBOXYLIC ACID (BENZYL-CYANO-METHYL)-AMIDES INHIBITORS OF CATHEPSIN C | Not Available | 14-
Mar- 13 |
19-
Dec -19 |
7-
May -20 |
| US20200131
518 |
CONTROL OF TOTAL AFUCOSYLATED GLYCOFORMS OF ANTIBODIES PRODUCED IN CELL CULTURE | Not Available | 14-
Mar- 17 |
14-
Mar -18 |
30-
Apr- 20 |
| US20200129
487 |
CHEMOTHERAPY FOR CANCER USING AZABICYCLO COMPOUND | TAIHO PHARMACEUTICAL CO., LTD. | 30-
Jun- 17 |
29-
Jun -18 |
30-
Apr- 20 |
| US20200127
954 |
RNA TARGETING METHODS AND COMPOSITIONS | Salk Institute for Biological Studies | 22-
Aug- 17 |
31-
Dec -18 |
23-
Apr- 20 |
| US20200124
599 |
ACCURATE, RAPID AND CONVENIENT SINGLE-STEP DISEASE DIAGNOSTIC METHOD USING SELF-AMPLIFICATION PRINCIPLE OF DETECTION SIGNAL | CELLEMEDY CO., LTD | 13-
Apr- 17 |
13-
Apr -18 |
23-
Apr- 20 |
| US20200123
233 |
AMINO ACID SEQUENCES DIRECTED AGAINST ENVELOPE PROTEINS OF A VIRUS AND POLYPEPTIDES COMPRISING THE SAME FOR THE TREATMENT OF VIRAL DISEASES | Ablynx N.V. | 5-
Jun- 08 |
1-
Aug -19 |
23-
Apr- 20 |
| US20200123
205 |
Inhibition Of TCR Signaling With Peptide Variants | Not Available | 22-
Oct- 18 |
18-
Dec -19 |
23-
Apr- 20 |
| US20200123
203 |
COMPOSITIONS COMPRISING CURONS AND USES THEREOF | FLAGSHIP PIONEERING INNOVATIONS V, INC. | 13-
Jun- 17 |
13-
Jun -18 |
23-
Apr- 20 |
| US20200115
448 |
CELLS EXPRESSING CHIMERIC ACTIVATING RECEPTORS AND CHIMERIC STIMULATING RECEPTORS AND USES THEREOF | Not Available | 26-
Apr- 17 |
22-
Oct -19 |
16-
Apr- 20 |
| US20200115
434 |
ANTIBODY/T-CELL RECEPTOR CHIMERIC CONSTRUCTS AND USES THEREOF | Not Available | 23-
Oct- 15 |
29-
Oct -19 |
16-
Apr- 20 |
| US20200113
996 |
CHIMERIC VIRUS-LIKE PARTICLES AND USES THEREOF AS ANTIGEN-SPECIFIC REDIRECTORS OF IMMUNE RESPONSES | Not Available | 23-
Jun- 17 |
21-
Jun -18 |
16-
Apr- 20 |
| US20200113
967 |
PEPTIDES AND USES THEREFOR AS ANTIVIRAL AGENTS | Not Available | 26-
May- 17 |
26-
May -17 |
16-
Apr- 20 |
| US20200113
831 |
LIPOSOMES HAVING USEFUL N:P RATIO FOR DELIVERY OF RNA MOLECULES | GLAXOSMITHKLINE BIOLOGICALS SA | 6-Jul-
11 |
16-
Dec -19 |
16-
Apr- 20 |
| US20200113
830 |
PEGYLATED LIPOSOMES FOR DELIVERY OF IMMUNOGEN-ENCODING RNA | GLAXOSMITHKLINE BIOLOGICALS S.A. | 31-
Aug- 11 |
16-
Dec -19 |
16-
Apr- 20 |
| US20200109
403 |
IN VIVO DELIVERY OF OLIGONUCLEOTIDES | Not Available | 12-
Dec- 11 |
18-
Dec -19 |
9-
Apr- 20 |
| US20200108
136 |
METHODS AND COMPOSITIONS FOR LIVE ATTENUATED VIRUSES | Not Available | 6-
Apr- 07 |
22-
Nov -19 |
9-
Apr- 20 |
| US20200102
558 |
TRANSKINGDOM PLATFORM FOR THERAPEUTIC NUCLEIC ACID DELIVERY | Not Available | 3-
Apr- 17 |
3-
Oct -19 |
2-
Apr- 20 |
| US20200102
550 |
TAL EFFECTOR-MEDIATED DNA MODIFICATION | Not Available | 10-
Dec- 09 |
28-
Aug -19 |
2-
Apr- 20 |
| US20200102
544 |
POXVIRUS-PLASMODIUM RECOMBINANTS, COMPOSITIONS CONTAINING SUCH RECOMBINANTS, USES THEREOF, AND METHODS OF MAKING AND USING THE SAME | Not Available | 30-
Dec- 13 |
11-
Sep -19 |
2-
Apr- 20 |
| US20200102
362 |
TUMOR NECROSIS FACTOR RECEPTOR (TNFR) BINDING PROTEIN COMPLEX WITH IMPROVED BINDING AND BIOACTIVITY | Not Available | 6-
Apr- 17 |
5-
Apr -18 |
2-
Apr- 20 |
| US20200102
292 |
SUBSTITUTED BENZOFURANYL AND BENZOXAZOLYL COMPOUNDS AND USES THEREOF | Not Available | 3-Jul-
13 |
10-
Jul- 19 |
2-
Apr- 20 |
| US20200101
158 |
ANTI-TIGIT ANTIGEN-BINDING PROTEINS AND METHODS OF USE THEREOF | Not Available | 1-
Oct- 15 |
22-
Oct -19 |
2-
Apr- 20 |
| US20200101
153 |
MVA-BN AND AD26.ZEBOV OR AD26.FILO PRIME-BOOST REGIMEN | Not Available | 6-
Apr- 17 |
6-
Apr -18 |
2-
Apr- 20 |
| US20200101
142 |
PDE5 COMPOSITIONS AND METHODS FOR IMMUNOTHERAPY | Not Available | 12-
Jun- 17 |
12-
Jun -18 |
2-
Apr- 20 |
| US20200101
119 |
METHODS OF TREATMENT OF INFECTIONS USING BACTERIA | Not Available | 27-
Sep- 18 |
26-
Sep -19 |
2-
Apr- 20 |
| US20200101
087 |
PHARMACEUTICAL COMPOSITIONS AND METHODS | Not Available | 3-
Aug- 15 |
21-
Nov -19 |
2-
Apr- 20 |
| US20200100
480 |
TRAIT SELECTION IN AVIANS | Not Available | 31-
May- 17 |
31-
May -18 |
2-
Apr- 20 |
| US20200095
324 |
ANTI-TIGIT ANTIGEN-BINDING PROTEINS AND METHODS OF USE THEREOF | Not Available | 30-
Mar- 17 |
30-
Mar -18 |
26-
Mar -20 |
| US20200093
919 |
MODIFIED PEDV SPIKE PROTEIN | Not Available | 20-
Sep- 18 |
18-
Sep -19 |
26-
Mar -20 |
| US20200093
909 |
PLASMODIUM SPOROZOITE NPDP PEPTIDES AS VACCINE AND TARGET NOVEL MALARIA VACCINES AND ANTIBODIES BINDING TO | Not Available | 19-
Apr- 17 |
19-
Apr -18 |
26-
Mar -20 |
| US20200093
855 |
ENHANCED IMMUNE RESPONSE UPON TREATMENT WITH NITRIC OXIDE | Not Available | 11-
Sep- 17 |
3-
Jun -19 |
26-
Mar -20 |
| US20200093
841 |
Broad Spectrum Antiviral and Methods of Use | Not Available | 17-
Apr- 06 |
30-
Apr -19 |
26-
Mar -20 |
| US20200087
655 |
PURIFICATION OF NUCLEIC ACIDS USING COPPER-TITANIUM OXIDES | Not Available | 14-
Jul-15 |
22-
Nov -19 |
19-
Mar -20 |
| US20200087
646 |
OPTIMIZED HUMAN CLOTTING FACTOR IX GENE EXPRESSION CASSETTES AND THEIR USE | Not Available | 31-
May- 17 |
31-
May -18 |
19-
Mar -20 |
| US20200087
630 |
INFLUENZA VIRUS AND TYPE 1 DIABETES | Istituto Zooprofilattico Sperimentale delle Venezie | 10-
Oct- 12 |
25-
Jul- 19 |
19-
Mar -20 |
| US20200087
359 |
GRIFFITHSIN MUTANTS | The United States of America,as represented by the Secretary,Department of Health and Human Services | 10-
Feb- 15 |
27-
Nov -19 |
19-
Mar -20 |
| US20200087
313 |
NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 29-
Jul-11 |
25-
Apr -19 |
19-
Mar -20 |
| US20200087
298 |
Imidazo[4,5-c] Ring Compounds Containing Guanidine Substituted Benzamide Groups | Not Available | 1-
Mar- 17 |
27-
Feb -18 |
19-
Mar -20 |
| US20200087
280 |
QUINAZOLINONES AND AZAQUINAZOLINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
25-
Nov -19 |
19-
Mar -20 |
| US20200086
324 |
DEVICES AND METHODS FOR NUCLEIC ACID EXTRACTION | Not Available | 30-
Jun- 16 |
27-
Dec -18 |
19-
Mar -20 |
| US20200085
984 |
APPARATUS, METHOD AND SYSTEM FOR SELECTIVELY AFFECTING AND/OR KILLING A VIRUS | THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK | 7-
Mar- 11 |
25-
Nov -19 |
19-
Mar -20 |
| US20200085
947 |
VISTA MODULATORS FOR DIAGNOSIS AND TREATMENT OF CANCER | Not Available | 7-
Sep- 12 |
14-
May -19 |
19-
Mar -20 |
| US20200085
943 |
PHARMACEUTICAL COMPOSITION COMPRISING A POLYMERIC CARRIER CARGO COMPLEX AND AT LEAST ONE PROTEIN OR PEPTIDE ANTIGEN | CureVac AG | 31-
Jan- 12 |
29-
Aug -19 |
19-
Mar -20 |
| US20200085
872 |
IMMUNE CELLS EXPRESSING ENGINEERED ANTIGEN RECEPTORS | Not Available | 19-
Apr- 17 |
19-
Apr -18 |
19-
Mar -20 |
| US20200085
852 |
EPIDERMAL MRNA VACCINE | Not Available | 5-
Aug- 15 |
5-
Aug -16 |
19-
Mar -20 |
| US20200085
756 |
NANOPARTICLE VACCINE ADJUVANT AND METHODS OF USE THEREOF | Not Available | 14-
Sep- 18 |
12-
Jul- 19 |
19-
Mar -20 |
| US20200080
141 |
METHODS AND COMPOSITIONS FOR ENRICHMENT OF AMPLIFICATION PRODUCTS | Not Available | 11-
Dec- 13 |
19-
Nov -19 |
12-
Mar -20 |
| US20200080
111 |
Methods for Autocatalytic Genome Editing and Neutralizing Autocatalytic Genome Editing and Compositions Thereof | Not Available | 18-
Sep- 15 |
19-
Sep -16 |
12-
Mar -20 |
| US20200079
820 |
DERIVATIVES OF AMANITA TOXINS AND THEIR CONJUGATION TO A CELL BINDING MOLECULE | Hangzhou DAC Biotech Co., Ltd. | 20-
Apr- 16 |
20-
Apr -16 |
12-
Mar -20 |
| US20200079
781 |
Substituted 2,4 diamino-quinoline as new medicament for fibrosis, autophagy and cathepsins B (CTSB), L (CTSL) and D (CTSD) related diseases | Not Available | 5-
Sep- 18 |
1-
Aug -19 |
12-
Mar -20 |
| US20200078
335 |
COMPOSITIONS AND METHODS TO REDUCE PATHOGENESIS | Not Available | 1-
May- 17 |
1-
May -18 |
12-
Mar -20 |
| US20200072
836 |
MEDIA ELABORATED WITH NEWLY SYNTHESIZED ANTIBODIES (MENSA) AND USES THEREOF | Not Available | 5-
May- 14 |
2-
Apr -19 |
5-
Mar -20 |
| US20200071
723 |
RNA-BASED DELIVERY SYSTEMS WITH LEVELS OF CONTROL | Not Available | 30-
Aug- 18 |
29-
Aug -19 |
5-
Mar -20 |
| US20200071
421 |
BISPECIFIC ANTIBODY | Not Available | 20-
Nov- 13 |
8-
Apr -19 |
5-
Mar -20 |
| US20200069
814 |
CONJUGATION OF A CYTOTOXIC DRUG WITH BIS-LINKAGE | Hangzhou DAC Biotech Co., Ltd. | 6-
Apr- 17 |
6-
Apr -17 |
5-
Mar -20 |
| US20200062
764 |
ALKYL PYRROLOPYRIMIDINE ANALOGS AND METHODS OF MAKING AND USING SAME | Not Available | 17-
Nov- 16 |
17-
Nov -17 |
27-
Feb -20 |
| US20200061
187 |
METHODS FOR PREPARING SQUALENE | Not Available | 12-
May- 10 |
4-
Nov -19 |
27-
Feb -20 |
| US20200061
185 |
PREFUSION CORONAVIRUS SPIKE PROTEINS AND THEIR USE | The Scripps Research Institute | 25-
Oct- 16 |
25-
Oct -17 |
27-
Feb -20 |
| US20200060
981 |
CATIONIC NANOPARTICLES FOR ENHANCING INFECTIOUS CAPACITY OF LIVE VIRUSES | Not Available | 9-
Dec- 16 |
11-
Dec -17 |
27-
Feb -20 |
| US20200056
221 |
METHODS FOR DIAGNOSING INFECTIOUS DISEASES USING ADSORPTION MEDIA | Not Available | 8-
Nov- 13 |
25-
Oct -19 |
20-
Feb -20 |
| US20200056
159 |
NOVEL ADENO-ASSOCIATED VIRUS (AAV) CLADE F VECTOR AND USES THEREFOR | Not Available | 28-
Feb- 17 |
27-
Feb -18 |
20-
Feb -20 |
| US20200055
927 |
MAST CELL STABILIZERS FOR TREATMENT OF HYPERCYTOKINEMIA AND VIRAL INFECTION | Not Available | 8-
Sep- 16 |
25-
Oct -19 |
20-
Feb -20 |
| US20200055
926 |
MAST CELL STABILIZERS FOR TREATMENT OF HYPERCYTOKINEMIA AND VIRAL INFECTION | Not Available | 8-
Sep- 16 |
25-
Oct -19 |
20-
Feb -20 |
| US20200054
731 |
IMMUNOMODULATORY COMPOSITIONS AND METHODS OF USE THEREOF | Not Available | 7-
Jan- 14 |
15-
Jul- 19 |
20-
Feb -20 |
| US20200054
660 |
DNA METHYLATION PROFILING FOR T-CELL IMMUNOTHERAPY | St. Jude Children’s Research Hospital | 9-
Dec- 16 |
7-
Dec -17 |
20-
Feb -20 |
| US20200054
259 |
HIGH DENSITY ANALOG MULTIPEXING | EnLiSense, LLC | 17-
Aug- 18 |
16-
Aug -19 |
20-
Feb -20 |
| US20200048
722 |
METHODS FOR REAL-TIME MULTIPLEX ISOTHERMAL DETECTION AND IDENTIFICATION OF BACTERIAL, VIRAL, AND PROTOZOAN NUCLEIC ACIDS | Not Available | 8-
May- 15 |
24-
Aug -19 |
13-
Feb -20 |
| US20200048
649 |
VIRUS-LIKE PARTICLES AND USES THEREOF | Not Available | 13-
Mar- 17 |
13-
Mar -18 |
13-
Feb -20 |
| US20200048
636 |
IMMUNISATION OF LARGE MAMMALS WITH LOW DOSES OF RNA | GLAXOSMITHKLINE BIOLOGICALS SA | 6-Jul-
10 |
18-
Oct -19 |
13-
Feb -20 |
| US20200046
865 |
DECONTAMINATION DEVICE AND METHOD USING ULTRASONIC CAVITATION | Not Available | 29-
Dec- 17 |
22-
Aug -19 |
13-
Feb -20 |
| US20200046
826 |
METHODS AND COMPOSITIONS FOR IMMUNIZATION AGAINST VIRUS | Academia Sinica | 27-
Mar- 09 |
4-
Jun -19 |
13-
Feb -20 |
| US20200046
692 |
SPECIFIC AKT3 INHIBITOR AND USES THEREOF | Not Available | 15-
Jan- 16 |
2-
Oct -19 |
13-
Feb -20 |
| US20200040
408 |
HANDHELD NUCLEIC ACID-BASED ASSAY FOR RAPID IDENTIFICATION | Not Available | 8-
Feb- 16 |
6-
May -19 |
6-
Feb -20 |
| US20200040
042 |
CHIMERIC MOLECULES AND USES THEREOF | Not Available | 30-
Mar- 17 |
29-
Mar -18 |
6-
Feb -20 |
| US20200038
871 |
DEVICES, PROCESSES, AND SYSTEMS FOR DETERMINATION OF NUCLEIC ACID SEQUENCE, EXPRESSION, COPY NUMBER, OR METHYLATION CHANGES USING COMBINED NUCLEASE, LIGASE, POLYMERASE, AND SEQUENCING REACTIONS | Not Available | 29-
Mar- 17 |
29-
Mar -18 |
6-
Feb -20 |
| US20200038
373 |
NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 9-
May- 12 |
16-
May -19 |
6-
Feb -20 |
| US20200033
343 |
EXOSOME-MEDIATED DIAGNOSIS OF HEPATITIS VIRUS INFECTIONS AND DISEASES | Not Available | 6-
Oct- 08 |
14-
Oct -19 |
30-
Jan- 20 |
| US20200032
255 |
METHODS AND COMPOSITIONS FOR THE TREATMENT OF CANCER OR OTHER DISEASES | Not Available | 26-
Jan- 07 |
6-
Mar -19 |
30-
Jan- 20 |
| US20200031
871 |
NOVEL DEPSIPEPTIDES AND USES THEREOF | Not Available | 4-
Apr- 17 |
30-
Mar -18 |
30-
Jan- 20 |
| US20200031
819 |
COMPOSITIONS AND METHODS FOR INHIBITING KINASES | Not Available | 23-
Apr- 15 |
26-
Jun -19 |
30-
Jan- 20 |
| US20200030
441 |
Lipidated Immune Response Modifier Compound Compositions, Formulations, and Methods | Not Available | 17-
Aug- 10 |
8-
Jul- 19 |
30-
Jan- 20 |
| US20200030
432 |
ZOONOTIC DISEASE RNA VACCINES | ModernaTX, Inc. | 17-
Mar- 17 |
16-
Mar -18 |
30-
Jan- 20 |
| US20200030
422 |
COMBINATION OF VACCINATION AND OX40 AGONISTS | CureVac AG | 9-
Sep- 16 |
15-
Apr -19 |
30-
Jan- 20 |
| US20200024
616 |
NOVEL RECOMBINANT ADENO-ASSOCIATED VIRUS CAPSIDS WITH ENHANCED HUMAN PANCREATIC TROPISM | Not Available | 30-
Mar- 18 |
29-
Mar -19 |
23-
Jan- 20 |
| US20200024
310 |
NOVEL DEPSIPEPTIDE AND USES THEREOF | Not Available | 3-
Dec- 12 |
1-
Aug -19 |
23-
Jan- 20 |
| US20200020
420 |
Method for Establishing Machine Learning Model for Predicting Toxicity of siRNA to Certain Type of Cells and Application Thereof | Not Available | 8-
Dec- 16 |
7-
Dec -17 |
16-
Jan- 20 |
| US20200017
926 |
CELL-FREE NUCLEIC ACIDS FOR THE ANALYSIS OF THE HUMAN MICROBIOME AND COMPONENTS THEREOF | Not Available | 7-
Nov- 13 |
28-
Aug -19 |
16-
Jan- 20 |
| US20200017
832 |
COMPOSITIONS FOR REPROGRAMMING CELLS INTO DENDRITIC CELLS OR ANTIGEN PRESENTING CELLS, METHODS AND USES THEREOF | Not Available | 5-
Apr- 17 |
5-
Apr -18 |
16-
Jan- 20 |
| US20200017
588 |
MODULAR TETRAVALENT BISPECIFIC ANTIBODY PLATFORM | Not Available | 14-
Oct- 16 |
16-
Oct -17 |
16-
Jan- 20 |
| US20200017
554 |
SELF-ASSEMBLING PROTEIN NANOPARTICLES WITH BUILT-IN SIX- HELIX BUNDLE PROTEINS | Not Available | 23-
Mar- 17 |
22-
Mar -18 |
16-
Jan- 20 |
| US20200017
514 |
ADAMANTANE DERIVATIVES FOR THE TREATMENT OF FILOVIRUS INFECTION | Not Available | 12-
Jul-18 |
12-
Jul- 18 |
16-
Jan- 20 |
| US20200017
455 |
CERTAIN (2S)-N-[(1S)-1-CYANO-2-PHENYLETHYL]-1,4- OXAZEPANE-2-CARBOXAMIDES AS DIPEPTIDYL PEPTIDASE 1 INHIBITORS | Not Available | 24-
Jan- 14 |
19-
Mar -19 |
16-
Jan- 20 |
| US20200016
589 |
LOADING VIALS | Not Available | 10-
Nov- 11 |
24-
Sep -19 |
16-
Jan- 20 |
| US20200016
286 |
Production of Immune-Response-Stimulating Aerosols By NonThermal Plasma Treatment Of Airborne Pathogens | Not Available | 13-
Jul-18 |
12-
Jul- 19 |
16-
Jan- 20 |
| US20200016
280 |
Compositions For Enhancing Transport Of Molecules Into Cells | Not Available | 29-
Apr- 03 |
9-
Apr -19 |
16-
Jan- 20 |
| US20200016
161 |
METHODS FOR TREATING VIRAL DISORDERS | TRUSTEES OF BOSTON UNIVERSITY | 24-
Sep- 09 |
22-
Jul- 19 |
16-
Jan- 20 |
| US20200010
883 |
METHODS AND COMPOSITIONS FOR ENRICHMENT OF AMPLIFICATION PRODUCTS | Not Available | 9-
Oct- 15 |
7-
Jun -19 |
9-
Jan- 20 |
| US20200010
519 |
NUCLEASE FUSIONS FOR ENHANCING GENOME EDITING BY HOMOLOGY-DIRECTED TRANSGENE INTEGRATION | Not Available | 10-
Mar- 17 |
9-
Mar -18 |
9-
Jan- 20 |
| US20200009
244 |
NANOPARTICLE VACCINES WITH NOVEL STRUCTURAL COMPONENTS | Not Available | 13-
Jun- 18 |
13-
Jun -19 |
9-
Jan- 20 |
| US20200002
674 |
ERYTHROID CELLS COMPRISING PHENYLALANINE HYDROXYLASE | Not Available | 18-
Nov- 13 |
16-
Sep -19 |
2-
Jan- 20 |
| US20200000
931 |
SYNTHETIC NANOPARTICLES FOR DELIVERY OF IMMUNOMODULATORY COMPOUNDS | Not Available | 15-
Jul-16 |
10-
Apr -19 |
2-
Jan- 20 |
| US20190391
150 |
COMPOSITIONS AND METHODS FOR CAPTURING EXOSOMES | Not Available | 1-
May- 15 |
10-
Sep -19 |
26-
Dec -19 |
| US20190390
249 |
BIOLOGICAL SPECIMEN COLLECTION AND TRANSPORT SYSTEM | Longhorn Vaccines and Diagnostics, LLC | 1-
Oct- 07 |
19-
Jun -17 |
26-
Dec -19 |
| US20190390
229 |
GENE EDITING REAGENTS WITH REDUCED TOXICITY | Not Available | 21-
Apr- 16 |
20-
Apr -17 |
26-
Dec -19 |
| US20190390
179 |
SYNTHETIC REVERSE TRANSCRIPTASES AND USES THEREOF | Not Available | 12-
Apr- 16 |
12-
Apr -17 |
26-
Dec -19 |
| US20190390
176 |
NOVEL RECOMBINANT ADENO-ASSOCIATED VIRUS CAPSIDS WITH ENHANCED HUMAN SKELETAL MUSCLE TROPISM | Not Available | 2-
Dec- 15 |
3-
Jul- 19 |
26-
Dec -19 |
| US20190389
816 |
ANTIVIRAL COMPOUNDS AND METHODS | Biotron Limited | 26-
Jun- 03 |
29-
Aug -19 |
26-
Dec -19 |
| US20190388
473 |
METHODS AND COMPOSITIONS FOR IMMUNOMODULATION | Not Available | 1-
Apr- 14 |
30-
Aug -19 |
26-
Dec -19 |
| US20190382
799 |
VIRAL METHODS OF MAKING GENETICALLY MODIFIED CELLS | Not Available | 27-
Oct- 16 |
19-
Apr -19 |
19-
Dec -19 |
| US20190382
433 |
GLYCOLIPIDS AND PHARMACEUTICAL COMPOSITIONS THEREOF FOR USE IN THERAPY | THE UNIVERSITY OF NOTTINGHAM | 4-
Apr- 14 |
25-
Jul- 19 |
19-
Dec -19 |
| US20190381
180 |
HYBRID CARRIERS FOR NUCLEIC ACID CARGO | Not Available | 9-
Jun- 16 |
9-
Jun -17 |
19-
Dec -19 |
| US20190381
162 |
PAN FILOVIRUS VACCINE COMPOSITIONS AND METHODS OF MAKING | Not Available | 28-
Mar- 16 |
27-
Mar -17 |
19-
Dec -19 |
| US20190381
155 |
COMBINATION OF VACCINATION AND INHIBITION OF THE PD-1 PATHWAY | CureVac AG | 22-
Feb- 13 |
29-
Aug -19 |
19-
Dec -19 |
| US20190380
995 |
PREVENTION AND TREATMENT OF VIRAL INFECTIONS | Not Available | 2-
Jun- 16 |
3-
Jul- 19 |
19-
Dec -19 |
| US20190380
891 |
MOBILE CLINICS | Baylor College of Medicine | 12-
Nov- 14 |
28-
Aug -19 |
19-
Dec -19 |
| US20190376
151 |
MODIFIED OLIGONUCLEOTIDES COMPRISING THIOL FUNCTIONS AND USE THEREOF FOR DETECTING NUCLEIC ACIDS | Not Available | 4-
Apr- 12 |
8-
Feb -19 |
12-
Dec -19 |
| US20190376
034 |
PLATELETS COMPRISING EXOGENOUS POLYPEPTIDES AND USES THEREOF | Not Available | 18-
Nov- 13 |
23-
Aug -19 |
12-
Dec -19 |
| US20190375
801 |
HSP FUSION PROTEIN WITH ANTI-CHEMOREPELLANT AGENT FOR TREATMENT OF INFECTIOUS DISEASE | Not Available | 9-
Sep- 16 |
8-
Sep -17 |
12-
Dec -19 |
| US20190374
650 |
COMPOSITIONS AND METHODS FOR DELIVERY OF POLYMER/BIOMACROMOLECULE CONJUGATES | Not Available | 22-
Feb- 17 |
21-
Feb -18 |
12-
Dec -19 |
| US20190374
610 |
MEDICAL USE OF INTERFERON-LAMBDA FOR THE TREATMENT OF FIBROSIS | Not Available | 20-
Dec- 16 |
20-
Dec -17 |
12-
Dec -19 |
| US20190374
576 |
VIRAL METHODS OF T CELL THERAPY | Not Available | 27-
Oct- 16 |
19-
Apr -19 |
12-
Dec -19 |
| US20190370
834 |
SYSTEM FOR DETERMINING PUBLIC SENTIMENT TOWARDS PATHOGENS | Not Available | 1-
Jun- 18 |
1-
Jun -18 |
5-
Dec -19 |
| US20190367
553 |
COMPOSITIONS AND METHODS OF MODULATING THE IMMUNE RESPONSE BY ACTIVATING ALPHA PROTEIN KINASE 1 | Not Available | 27-
Oct- 17 |
26-
Apr -19 |
5-
Dec -19 |
| US20190367
525 |
PYRROLO AND PYRAZOLOPYRIMIDINES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 30-
Dec- 14 |
19-
Jul- 19 |
5-
Dec -19 |
| US20190367
453 |
CONJUGATES OF CELL BINDING MOLECULES WITH CYTOTOXIC AGENTS | HANGZHOU DAC BIOTECH CO., LTD. | 12-
Jul-12 |
13-
Aug -19 |
5-
Dec -19 |
| US20190365
925 |
AAV VECTORS TARGETED TO THE CENTRAL NERVOUS SYSTEM | Not Available | 21-
Nov- 14 |
13-
Jun -19 |
5-
Dec -19 |
| US20190365
756 |
PYRIMIDINE COMPOUNDS CONTAINING ACIDIC GROUPS | Not Available | 4-
Jun- 18 |
3-
Jun -19 |
5-
Dec -19 |
| US20190359
990 |
VIRAL SYNTHETIC NUCLEIC ACID SEQUENCES AND USE THEREOF | Not Available | 25-
Jan- 17 |
25-
Jan -18 |
28-
Nov -19 |
| US20190359
635 |
ISOTHIAZOLOPYRIMIDINONES, PYRAZOLOPYRIMIDINONES, AND PYRROLOPYRIMIDINONES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
18-
Jul- 19 |
28-
Nov -19 |
| US20190359
629 |
THIENOPYRIMIDINONES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
18-
Jul- 19 |
28-
Nov -19 |
| US20190358
335 |
STOMACH ACID-STABLE AND MUCIN-BINDING PROTEIN-POLYMER CONJUGATES | Not Available | 12-
Jan- 17 |
12-
Jan -18 |
28-
Nov -19 |
| US20190358
312 |
ANTIGEN-ADJUVANT COUPLING REAGENTS AND METHODS OF USE | Not Available | 19-
Dec- 17 |
19-
Dec -18 |
28-
Nov -19 |
| US20190358
304 |
ORAL DELIVERY OF ANGIOTENSIN CONVERTING ENZYME 2 (ACE2) OR ANGIOTENSIN-(1-7)-BIOENCAPSULATED IN PLANT CELLS ATTENUATES PULMONARY HYPERTESNIONS, CARDIAC DYSFUNCTION AND DEVELOPMENT OF AUTOIMMUNE AND EXPOERIMENTALLY INDUCED OCULAR DISORDERS | Not Available | 18-
Oct- 13 |
29-
May -19 |
28-
Nov -19 |
| US20190358
170 |
Lipids and Lipid Compositions for the Delivery of Active Agents | Not Available | 19-
Dec- 13 |
12-
Aug -19 |
28-
Nov -19 |
| US20190352
639 |
GENOME EDITING REAGENTS AND THEIR USE | Not Available | 10-
Feb- 17 |
12-
Feb -18 |
21-
Nov -19 |
| US20190352
615 |
METHOD FOR PURIFYING VIRUS | Not Available | 22-
Dec- 16 |
21-
Dec -17 |
21-
Nov -19 |
| US20190352
608 |
CD137 ENRICHMENT FOR EFFICIENT TUMOR INFILTRATING LYMPHOCYTE SELECTION | Not Available | 16-
Sep- 13 |
30-
Jan -19 |
21-
Nov -19 |
| US20190352
357 |
HELIX-GRAFTED PROTEINS AS INHIBITORS OF DISEASE-RELEVANT PROTEIN-PROTEIN INTERACTIONS | Colorado State University Research Foundation | 7-
Nov- 14 |
7-
Aug -19 |
21-
Nov -19 |
| US20190346
443 |
EXOSOME-MEDIATED DIAGNOSIS OF HEPATITIS VIRUS INFECTIONS AND DISEASES | Not Available | 6-
Oct- 08 |
28-
Jun -19 |
14-
Nov -19 |
| US20190345
504 |
ARTIFICIAL NUCLEIC ACID MOLECULES | CureVac AG | 30-
Dec- 14 |
26-
Jul- 19 |
14-
Nov -19 |
| US20190345
503 |
CIRCULAR RNAS AND THEIR USE IN IMMUNOMODULATION | Not Available | 20-
Jun- 16 |
15-
Jun -17 |
14-
Nov -19 |
| US20190345
481 |
PURIFICATION OF NUCLEIC ACIDS USING METAL-TITANIUM OXIDES | Not Available | 14-
Jul-15 |
5-
Jul- 19 |
14-
Nov -19 |
| US20190345
221 |
BROAD SPECTRUM VACCINE, PREPARING METHOD AND APPLICATION THEREOF | Tianjin Dongya Biological Technology Co., Ltd. | 9-
May- 18 |
9-
May -18 |
14-
Nov -19 |
| US20190345
166 |
COMPOUNDS AND COMPOSITIONS AS TOLL-LIKE RECEPTOR 7 AGONISTS | Not Available | 1-
May- 14 |
24-
Jul- 19 |
14-
Nov -19 |
| US20190343
862 |
DELIVERY OF RNA TO TRIGGER MULTIPLE IMMUNE PATHWAYS | GLAXOSMITHKLINE BIOLOGICALS, SA | 6-Jul-
10 |
16-
Jul- 19 |
14-
Nov -19 |
| US20190337
990 |
POLYPEPTIDES FOR ENGINEERING INTEGRASE CHIMERIC PROTEINS AND THEIR USE IN GENE THERAPY | Not Available | 13-
Feb- 15 |
5-
Jun -19 |
7-
Nov -19 |
| US20190336
969 |
PARALLELIZED SAMPLE HANDLING | Not Available | 19-
Apr- 13 |
27-
Mar -19 |
7-
Nov -19 |
| US20190336
611 |
HYBRID CARRIERS FOR NUCLEIC ACID CARGO | CureVac AG | 9-
Jun- 16 |
9-
Jun -17 |
7-
Nov -19 |
| US20190336
608 |
CATIONIC CARRIERS FOR NUCLEIC ACID DELIVERY | CureVac AG | 9-
Jun- 16 |
9-
Jun -17 |
7-
Nov -19 |
| US20190336
597 |
METHODS OF GENERATING ROBUST PASSIVE AND ACTIVE IMMUNE RESPONSES | Not Available | 8-
Jan- 13 |
17-
May -19 |
7-
Nov -19 |
| US20190336
456 |
USE OF XIBORNOL AS ACTIVE AGENT IN THE TREATMENT OF VIRAL INFECTIONS | ABIOGEN PHARMA S.P.A. | 15-
Jul-16 |
13-
Jul- 17 |
7-
Nov -19 |
| US20190330
618 |
ENZYMATIC ENCODING METHODS FOR EFFICIENT SYNTHESIS OF LARGE LIBRARIES | Not Available | 1-
Dec- 05 |
22-
Jan -19 |
31-
Oct- 19 |
| US20190330
572 |
CLEANING COMPOSITION, METHOD OF MAKING AND USE THEREOF | Not Available | 8-
Sep- 16 |
21-
Jun -19 |
31-
Oct- 19 |
| US20190330
245 |
Boron-Containing Small Molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
12-
Jul- 19 |
31-
Oct- 19 |
| US20190330
187 |
Chemical Compounds | Not Available | 18-
Mar- 14 |
10-
May -19 |
31-
Oct- 19 |
| US20190330
164 |
HYDRAZIDE CONTAINING NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 29-
Jul-11 |
28-
Nov -18 |
31-
Oct- 19 |
| US20190330
149 |
CONJUGATES OF CELL BINDING MOLECULES WITH CYTOTOXIC AGENTS | HANGZHOU DAC BIOTECH CO., LTD. | 12-
Jul-12 |
9-
May -19 |
31-
Oct- 19 |
| US20190328
869 |
IMMUNOTHERAPEUTIC PRODUCT AND MDSC MODULATOR COMBINATION THERAPY | Transgene SA | 10-
Oct- 16 |
10-
Oct -17 |
31-
Oct- 19 |
| US20190328
865 |
IMMUNOGENIC COMPOSITION FOR MERS CORONAVIRUS INFECTION | Not Available | 28-
Jul-14 |
17-
Nov -17 |
31-
Oct- 19 |
| US20190328
804 |
AAV Vectors Targeted to Oligodendrocytes | Not Available | 28-
Sep- 12 |
15-
Jul- 19 |
31-
Oct- 19 |
| US20190323
068 |
PCR Ready Compositions and Methods for Screening Biological Samples | Longhorn Vaccines and Diagnostics, LLC | 12-
Sep- 06 |
28-
Jun -19 |
24-
Oct- 19 |
| US20190322
989 |
PRODUCTION OF VIRUSES IN CELL CULTURE | Not Available | 24-
Nov- 15 |
25-
Jan -19 |
24-
Oct- 19 |
| US20190322
725 |
COMPOSITIONS COMPRISING AAV EXPRESSING DUAL ANTIBODY CONSTRUCTS AND USES THEREOF | Not Available | 13-
May- 14 |
2-
Jul- 19 |
24-
Oct- 19 |
| US20190321
481 |
IMMUNOMODULATORY COMPOSITIONS, PROCESSES FOR MAKING THE SAME, AND METHODS FOR INHIBITING CYTOKINE STORMS | NantBio, Inc. | 11-
Nov- 16 |
9-
Nov -17 |
24-
Oct- 19 |
| US20190321
403 |
ENGINEERED B CELLS AND RELATED COMPOSITIONS AND METHODS | Juno Therapeutics, Inc. | 2-
Dec- 16 |
30-
Nov -17 |
24-
Oct- 19 |
| US20190316
109 |
COMPOSITION COMPRISING A GENE VECTOR THAT SELECTIVELY DEPLETES P16 POSITIVE SENESCENT CELLS | Not Available | 17-
Apr- 12 |
10-
Jun -19 |
17-
Oct- 19 |
| US20190316
091 |
ERYTHROID CELLS COMPRISING ARGINASE | Not Available | 18-
Nov- 13 |
30-
May -19 |
17-
Oct- 19 |
| US20190316
090 |
ERYTHROID CELLS COMPRISING ARGININE DEIMINASE | RUBIUS THERAPEUTICS, INC. | 18-
Nov- 13 |
30-
May -19 |
17-
Oct- 19 |
| US20190316
089 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
10-
May -19 |
17-
Oct- 19 |
| US20190315
840 |
ANTI-DENGUE VIRUS ANTIBODIES, POLYPEPTIDES CONTAINING VARIANT FC REGIONS, AND METHODS OF USE | Not Available | 16-
Sep- 16 |
10-
Jun -19 |
17-
Oct- 19 |
| US20190315
807 |
VIRUS-LIKE PARTICLES WITH HIGH-DENSITY COATING FOR INDUCING THE EXPRESSION OF ANTIBODIES | Not Available | 27-
Jul-16 |
26-
Jul- 17 |
17-
Oct- 19 |
| US20190314
496 |
POLYMERIC CARRIER CARGO COMPLEX FOR USE AS AN IMMUNOSTIMULATING AGENT OR AS AN ADJUVANT | CureVac AG | 1-
Apr- 14 |
18-
Jun -19 |
17-
Oct- 19 |
| US20190314
483 |
Vaccines Including Antigen From Four Strains of Influenza Virus | Not Available | 6-
Dec- 06 |
30-
Nov -18 |
17-
Oct- 19 |
| US20190314
482 |
VACCINES AGAINST INFECTIOUS DISEASES CAUSED BY POSITIVE STRANDED RNA VIRUSES | Medigen, Inc. | 28-
Nov- 16 |
21-
Nov -17 |
17-
Oct- 19 |
| US20190314
480 |
IMMUNOGENIC COMPOSITIONS AND USES THEREOF | Not Available | 25-
Mar- 15 |
23-
Apr -19 |
17-
Oct- 19 |
| US20190314
471 |
MOLECULAR VACCINES FOR INFECTIOUS DISEASE | Not Available | 2-
Oct- 08 |
27-
Jun -19 |
17-
Oct- 19 |
| US20190314
455 |
CYTOKINE CONJUGATES FOR THE TREATMENT OF PROLIFERATIVE AND INFECTIOUS DISEASES | Not Available | 3-
Aug- 17 |
7-
Jun -19 |
17-
Oct- 19 |
| US20190314
372 |
PYRIMIDINE COMPOUNDS CONTAINING ACIDIC GROUPS | Not Available | 5-
Dec- 16 |
25-
Mar -19 |
17-
Oct- 19 |
| US20190310
168 |
AIRBORNE AGENT COLLECTORS, METHODS, SYSTEMS AND DEVICES FOR MONITORING AIRBORNE AGENTS | Not Available | 22-
Jul-14 |
4-
Mar -19 |
10-
Oct- 19 |
| US20190309
357 |
CRISPR EFFECTOR SYSTEM BASED DIAGNOSTICS | MASSACHUSETTS INSTITUTE OF TECHNOLOGY | 9-
Dec- 16 |
25-
Feb -19 |
10-
Oct- 19 |
| US20190309
262 |
ERYTHROID CELLS COMPRISING SERINE DEHYDRATASE | Not Available | 18-
Nov- 13 |
4-
Jun -19 |
10-
Oct- 19 |
| US20190309
261 |
ERYTHROID CELLS COMPRISING LYSINE OXIDASE | Not Available | 18-
Nov- 13 |
4-
Jun -19 |
10-
Oct- 19 |
| US20190309
048 |
Optimized Human Clotting Factor VIII Gene Expression Cassettes and Their Use | Not Available | 6-
Feb- 15 |
20-
May -19 |
10-
Oct- 19 |
| US20190309
039 |
CRYPTIC POLYPEPTIDES AND USES THEREOF | Not Available | 29-
Jan- 15 |
22-
Apr -19 |
10-
Oct- 19 |
| US20190308
980 |
PYRROLOTRIAZINONES AND IMIDAZOTRIAZINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 30-
Dec- 14 |
24-
Jun -19 |
10-
Oct- 19 |
| US20190308
969 |
HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS | Not Available | 11-
Nov- 16 |
13-
Nov -17 |
10-
Oct- 19 |
| US20190308
943 |
3,5-DIAMINO-6-CHLORO-N-(N-(4-PHENYLBUTYL)CARBAMIMIDOYL) PYRAZINE-2- CARBOXAMIDE COMPOUNDS | Parion Sciences, Inc. | 17-
Dec- 12 |
13-
Mar -19 |
10-
Oct- 19 |
| US20190307
878 |
IMMUNE COMPLEX | The Rockefeller University | 20-
Mar- 15 |
10-
May -19 |
10-
Oct- 19 |
| US20190307
722 |
ANTIVIRAL COMPOSITIONS FOR THE TREATMENT OF INFECTIONS LINKED TO CORONAVIRUSES | Not Available | 21-
Oct- 16 |
20-
Oct -17 |
10-
Oct- 19 |
| US20190298
824 |
ALBUMIN-BINDING IMMUNOMODULATORY COMPOSITIONS AND METHODS OF USE THEREOF | THE UNITED STATES OF AMERICA, as represented by the Secretary, Department of Health and Human Serv | 4-
May- 16 |
4-
May -17 |
3-
Oct- 19 |
| US20190298
752 |
METHODS FOR THE USE OF 5′-ADENOSINE DIPHOSPHATE RIBOSE (ADPR) | Invirsa, Inc. | 27-
Mar- 18 |
26-
Mar -19 |
3-
Oct- 19 |
| US20190298
750 |
Nucleotide and Nucleoside Therapeutic Compositions and Uses Related Thereto | Not Available | 11-
Sep- 13 |
21-
Nov -18 |
3-
Oct- 19 |
| US20190293
656 |
NON-RADIOACTIVE CYTOTOXICITY ASSAYS | Not Available | 19-
Sep- 16 |
19-
Sep -17 |
26-
Sep -19 |
| US20190292
580 |
DNA LOGIC-GATED PROXIMITY ASSEMBLY CIRCUIT FOR BIOCHEMICAL SENSING | Rutgers, The State University of New Jersey | 23-
Mar- 18 |
22-
Mar -19 |
26-
Sep -19 |
| US20190292
563 |
ADENO-ASSOCIATED VIRUS (AAV) SEROTYPE 8 SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
8-
Apr -19 |
26-
Sep -19 |
| US20190292
561 |
SCALABLE METHODS FOR PRODUCING RECOMBINANT ADENO- ASSOCIATED VIRAL (AAV) VECTOR IN SERUM-FREE SUSPENSION CELL CULTURE SYSTEM SUITABLE FOR CLINICAL USE | SPARK THERAPEUTICS, INC. | 1-
Dec- 15 |
1-
Dec -16 |
26-
Sep -19 |
| US20190292
236 |
MODULATION OF IFI16 AND STING ACTIVITY | Not Available | 9-
Aug- 16 |
9-
Aug -17 |
26-
Sep -19 |
| US20190292
216 |
Cyclic Di-Nucleotide Induction of Type I Interferon | Not Available | 3-
May- 13 |
19-
Feb -19 |
26-
Sep -19 |
| US20190292
178 |
HOST TARGETED INHIBITORS OF DENGUE VIRUS AND OTHER VIRUSES | Dana-Farber Cancer Institute, Inc. | 11-
Apr- 12 |
26-
Jan -18 |
26-
Sep -19 |
| US20190290
674 |
Composition for Promoting Production of Immunostimulatory Factor | Kyoto University | 2-
Aug- 16 |
31-
Jul- 17 |
26-
Sep -19 |
| US20190285
632 |
METABALOMICS AND VIRAL DIAGNOSTICS SUITE | Excision Biotherapeutics, Inc. | 24-
May- 16 |
24-
May -17 |
19-
Sep -19 |
| US20190284
575 |
REVERSE GENETICS USING NON-ENDOGENOUS POL I PROMOTERS | Not Available | 21-
May- 09 |
30-
Jan -19 |
19-
Sep -19 |
| US20190284
531 |
DETECTION OF T CELL EXHAUSTION OR LACK OF T CELL COSTIMULATION AND USES THEREOF | Not Available | 15-
May- 15 |
24-
May -19 |
19-
Sep -19 |
| US20190284
230 |
ASSEMBLED GLYCOPROTEINS | Not Available | 29-
Sep- 16 |
22-
Sep -17 |
19-
Sep -19 |
| US20190282
694 |
IMMUNOPROTECTIVE PRIMARY MESENCHYMAL STEM CELLS AND METHODS | AUTOIMMUNE TECHNOLOGIES, LLC | 14-
Mar- 13 |
30-
May -19 |
19-
Sep -19 |
| US20190282
608 |
Method Of Treating Inflammation | Not Available | 1-
Apr- 10 |
29-
May -19 |
19-
Sep -19 |
| US20190276
523 |
COMPOSITION AND METHODS OF TREATING B CELL DISORDERS | Not Available | 2-
Sep- 16 |
5-
Sep -17 |
12-
Sep -19 |
| US20190275
519 |
SYSTEM FOR THE PRODUCTION OF CELLS AND/OR CELL PRODUCTS | Not Available | 8-
Nov- 16 |
8-
Nov -17 |
12-
Sep -19 |
| US20190275
101 |
STRUCTURE OF GII.4 NOROVIRUS PROTEASE – DESIGN OF BROAD- SPECTRUM PROTEASE INHIBITORS | Not Available | 5-
Oct- 16 |
5-
Oct -17 |
12-
Sep -19 |
| US20190269
694 |
HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS | Not Available | 8-
Mar- 11 |
11-
Oct -18 |
5-
Sep -19 |
| US20190269
672 |
Specific Akt3 Inhibitor and Uses Thereof | Not Available | 15-
Jan- 16 |
20-
May -19 |
5-
Sep -19 |
| US20190264
267 |
PHASING | Wave Life Sciences Ltd. | 25-
Jul-16 |
24-
Jul- 17 |
29-
Aug -19 |
| US20190264
177 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
10-
May -19 |
29-
Aug -19 |
| US20190263
934 |
FC VARIANTS WITH ENHANCED BINDING TO FCRN AND PROLONGED HALF-LIFE | Not Available | 26-
Jan- 18 |
25-
Jan -19 |
29-
Aug -19 |
| US20190262
448 |
Cationic Oil-In-Water Emulsions | GLAXOSMITHKLINE BIOLOGICALS, SA | 18-
Sep- 11 |
7-
Mar -19 |
29-
Aug -19 |
| US20190262
371 |
METHODS AND COMPOSITION FOR THE TREATMENT OF RNA VIRAL INFECTIONS | Not Available | 20-
Oct- 16 |
20-
Oct -17 |
29-
Aug -19 |
| US20190256
585 |
ANTIBODY SPECIFICALLY BINDING TO AN ISOLATED PEPTIDE DERIVED FROM VIMENTIN OR A FRAGMENT BINDING TO THE PEPTIDE | Not Available | 10-
Jun- 15 |
10-
Jun -16 |
22-
Aug -19 |
| US20190256
579 |
MIDDLE EAST RESPIRATORY SYNDROME CORONAVIRUS IMMUNOGENS, ANTIBODIES, AND THEIR USE | The U.S.A., as represented by the Secretary, Department of Health and Human Services | 24-
Feb- 15 |
3-
May -19 |
22-
Aug -19 |
| US20190255
085 |
METHODS FOR TREATING ARENAVIRIDAE AND CORONAVIRIDAE VIRUS INFECTIONS | Not Available | 16-
Sep- 15 |
1-
Feb -19 |
22-
Aug -19 |
| US20190254
968 |
OIL-IN-WATER EMULSIONS THAT CONTAIN NUCLEIC ACIDS | GLAXOSMITHKLINE BIOLOGICALS S.A. | 6-Jul-
11 |
2-
May -19 |
22-
Aug -19 |
| US20190250
153 |
MULTI-CONFIGURABLE SENSING ARRAY AND METHODS OF USING SAME | Board of Regents, The University of Texas System | 20-
Oct- 16 |
19-
Oct -17 |
15-
Aug -19 |
| US20190248
883 |
HUMAN MONOCLONAL ANTIBODIES AGAINST INTERLEUKIN 8 (IL-8) | Not Available | 16-
Dec- 02 |
20-
Feb -19 |
15-
Aug -19 |
| US20190248
866 |
POLYPEPTIDES AND USES THEREOF FOR TREATMENT OF AUTOIMMUNE DISORDERS AND INFECTION | Not Available | 30-
Jun- 11 |
30-
Apr -18 |
15-
Aug -19 |
| US20190248
865 |
ANTIBODY/T-CELL RECEPTOR CHIMERIC CONSTRUCTS AND USES THEREOF | Not Available | 23-
Oct- 15 |
21-
Oct -16 |
15-
Aug -19 |
| US20190247
529 |
METHOD AND SYSTEM FOR DECONTAMINATING SMALL ENCLOSURES | Not Available | 29-
Dec- 17 |
23-
Apr -19 |
15-
Aug -19 |
| US20190247
489 |
ADJUVANTED INFLUENZA B VIRUS VACCINES FOR PEDIATRIC PRIMING | Not Available | 20-
Oct- 11 |
16-
Nov -18 |
15-
Aug -19 |
| US20190247
485 |
Dimethyl Fumarate and Vaccination Regimens | Biogen MA Inc. | 14-
Mar- 14 |
23-
Apr -19 |
15-
Aug -19 |
| US20190247
440 |
METHODS AND COMPOSITIONS FOR IMMUNOMODULATION | Not Available | 1-
Apr- 14 |
12-
Apr -19 |
15-
Aug -19 |
| US20190247
367 |
TREATMENT OF INFECTIOUS DISEASES | CHILDREN’S MEDICAL CENTER CORPORATION | 26-
Jan- 15 |
23-
Apr -19 |
15-
Aug -19 |
| US20190241
646 |
ANTI-PNEUMOCOCCAL HYPERIMMUNE GLOBULIN FOR THE TREATMENT AND PREVENTION OF PNEUMOCOCCAL INFECTION | Not Available | 15-
Mar- 17 |
15-
Apr -19 |
8-
Aug -19 |
| US20190241
618 |
INHIBITION OF TCR SIGNALING WITH PEPTIDE VARIANTS | Not Available | 30-
Sep- 09 |
22-
Oct -18 |
8-
Aug -19 |
| US20190240
317 |
HPIV3 RNA VACCINES | ModernaTX, Inc. | 22-
Oct- 15 |
28-
Mar -19 |
8-
Aug -19 |
| US20190233
447 |
PROTEIN PROXIMITY ASSAY IN FORMALIN FIXED PARAFFIN EMBEDDED TISSUE USING CAGED HAPTENS | Not Available | 28-
Aug- 15 |
25-
Feb -19 |
1-
Aug -19 |
| US20190232
282 |
METHODS AND COMPOSITIONS FOR DETECTING ANALYTES | Not Available | 23-
Sep- 16 |
20-
Sep -17 |
1-
Aug -19 |
| US20190231
004 |
MASK | NBC MESHTEC INC. | 17-
Oct- 16 |
13-
Oct -17 |
1-
Aug -19 |
| US20190225
986 |
GENE TRANSFER INTO AIRWAY EPITHELIAL STEM CELL BY USING LENTIVIRAL VECTOR PSEUDOTYPED WITH RNA VIRUS OR DNA
VIRUS SPIKE PROTEIN |
Not Available | 28-
Oct- 05 |
30-
Nov -18 |
25-
Jul- 19 |
| US20190225
971 |
METHOD OF INCREASING THE REPLICATION OF A CIRCULAR DNA MOLECULE | Not Available | 10-
Aug- 15 |
4-
Aug -16 |
25-
Jul- 19 |
| US20190224
339 |
COMPOSITIONS FOR THE TREATMENT OF DISEASE | Not Available | 29-
Apr- 16 |
28-
Apr -17 |
25-
Jul- 19 |
| US20190223
445 |
ANTIMICROBIAL GEOPOLYMER COMPOSITIONS | Not Available | 14-
Jul-16 |
13-
Jul- 17 |
25-
Jul- 19 |
| US20190220
524 |
DETERMINING EXPLANATIONS FOR PREDICTED LINKS IN KNOWLEDGE GRAPHS | Not Available | 16-
Jan- 18 |
29-
Mar -18 |
18-
Jul- 19 |
| US20190219
563 |
Assay for Detecting TH1 and TH2 Cell Populations | Not Available | 16-
May- 14 |
17-
Dec -18 |
18-
Jul- 19 |
| US20190218
574 |
METHOD OF INCREASING THE FUNCTION OF AN AAV VECTOR | The Trustees of the University of Pennsylvania | 2-
Oct- 07 |
28-
Mar -19 |
18-
Jul- 19 |
| US20190218
277 |
ANTI-DENGUE VIRUS ANTIBODIES, POLYPEPTIDES CONTAINING VARIANT FC REGIONS, AND METHODS OF USE | Not Available | 16-
Sep- 16 |
15-
Sep -17 |
18-
Jul- 19 |
| US20190218
207 |
3-(Pyridin-3-yl)-Acrylamide and N-(Pyridin-3-yl)-Acrylamide Derivatives and Their Use as PAK or NaMPT Modulators | Not Available | 17-
Aug- 15 |
17-
Aug -16 |
18-
Jul- 19 |
| US20190216
951 |
METHOD FOR INCREASING EXPRESSION OF RNA-ENCODED PROTEINS | CureVac AG | 21-
Aug- 13 |
1-
Apr -19 |
18-
Jul- 19 |
| US20190216
917 |
HMPV RNA VACCINES | ModernaTX, Inc. | 22-
Oct- 15 |
28-
Mar -19 |
18-
Jul- 19 |
| US20190216
915 |
TRANSGENIC VERO-CD4/CCR5 CELL LINE | Not Available | 2-
Oct- 15 |
15-
Feb -19 |
18-
Jul- 19 |
| US20190216
841 |
Regimens and Compositions for AAV-Mediated Passive Immunization of Airborne Pathogens | Not Available | 20-
Apr- 11 |
20-
Mar -19 |
18-
Jul- 19 |
| US20190211
361 |
COMPOSITIONS COMPRISING CURONS AND USES THEREOF | Not Available | 13-
Jun- 17 |
27-
Mar -19 |
11-
Jul- 19 |
| US20190211
355 |
DNA MOLECULES PRODUCING CUSTOM DESIGNED REPLICATING AND NON-REPLICATING NEGATIVE STRANDED RNA VIRUSES AND USES THERE OF | Not Available | 5-
Jan- 15 |
4-
Jan -16 |
11-
Jul- 19 |
| US20190211
024 |
SMALL MOLECULES HAVING ANTIVIRAL PROPERTIES | General Research Laboratory | 19-
Aug- 16 |
19-
Aug -17 |
11-
Jul- 19 |
| US20190209
678 |
MANUFACTURE OF SURFACTANT-CONTAINING COMPOSITIONS WITH ENHANCED STABILITY | Not Available | 2-
Dec- 14 |
12-
Mar -19 |
11-
Jul- 19 |
| US20190209
604 |
OLIGONUCLEOTIDES, COMPOSITIONS AND METHODS THEREOF | WAVE LIFE SCIENCES LTD. | 3-
Jun- 16 |
2-
Jun -17 |
11-
Jul- 19 |
| US20190207
890 |
RNA TARGETING METHODS AND COMPOSITIONS | Salk Institute for Biological Studies | 22-
Aug- 17 |
31-
Dec -18 |
4-
Jul- 19 |
| US20190204
330 |
APPLICATION OF CLICK CHEMISTRY FOR SIGNAL AMPLIFICATION IN IHC AND ISH ASSAYS | Not Available | 28-
Jun- 16 |
19-
Dec -18 |
4-
Jul- 19 |
| US20190203
268 |
LOOP-MEDIATED ISOTHERMAL AMPLIFICATION (LAMP) BASED ASSAY FOR DETECTING MICROBES | Not Available | 2-
Jan- 18 |
31-
Dec -18 |
4-
Jul- 19 |
| US20190203
186 |
PRODUCTION OF VIRUSES IN AVIAN EGGS | Not Available | 24-
Nov- 15 |
25-
Jan -19 |
4-
Jul- 19 |
| US20190203
170 |
Avian Cells for Improved Virus Production | Not Available | 5-
Jun- 13 |
21-
Dec -18 |
4-
Jul- 19 |
| US20190202
929 |
COMPOSITIONS AND METHODS FOR IDENTIFICATION, ASSESSMENT, PREVENTION, AND TREATMENT OF AML USING USP10 BIOMARKERS AND MODULATORS | Not Available | 20-
Sep- 16 |
20-
Sep -17 |
4-
Jul- 19 |
| US20190202
868 |
CORONAVIRUS PROTEINS AND ANTIGENS | Phibro Animal Health Corporation | 7-
Feb- 14 |
15-
Mar -19 |
4-
Jul- 19 |
| US20190202
854 |
ENANTIOMERS OF THE 1′,6′-ISOMER OF NEPLANOCIN A | Not Available | 4-
Aug- 14 |
8-
Mar -19 |
4-
Jul- 19 |
| US20190201
565 |
DECONTAMINATION DEVICE AND METHOD USING ULTRASONIC CAVITATION | Not Available | 29-
Dec- 17 |
11-
Sep -18 |
4-
Jul- 19 |
| US20190201
564 |
DECONTAMINATION DEVICE AND METHOD USING ULTRASONIC CAVITATION | Not Available | 29-
Dec- 17 |
29-
Dec -17 |
4-
Jul- 19 |
| US20190201
552 |
Aptamer Compositions and Methods of Use Thereof | Not Available | 28-
Dec- 17 |
28-
Dec -18 |
4-
Jul- 19 |
| US20190201
433 |
2′-SUBSTITUTED-N6-SUBSTITUTED PURINE NUCLEOTIDES FOR RNA VIRUS TREATMENT | Atea Pharmaceuticals, Inc. | 7-
Sep- 16 |
5-
Mar -19 |
4-
Jul- 19 |
| US20190201
352 |
DESIGN, SYNTHESIS AND METHODS OF USE OF ACYCLIC FLEXMIER NUCLEOSIDE ANALOGUES HAVING ANTI-CORONAVIRUS ACTIVITY | Not Available | 30-
Jan- 15 |
12-
Mar -19 |
4-
Jul- 19 |
| US20190201
337 |
HYDROPHILIC FILTRATION DURING MANUFACTURE OF VACCINE ADJUVANTS | Not Available | 3-
Dec- 09 |
3-
Jan -19 |
4-
Jul- 19 |
| US20190194
728 |
Systemic inflammatory and pathogen biomarkers and uses therefor | Not Available | 24-
Aug- 16 |
24-
Aug -17 |
27-
Jun- 19 |
| US20190194
717 |
METHOD AND KIT FOR DETECTING PATHOGENIC MICROORGANISM | JAPAN SCIENCE AND TECHNOLOGY AGENCY | 5-
Sep- 16 |
4-
Sep -17 |
27-
Jun- 19 |
| US20190194
628 |
METHODS FOR PRODUCING VIRUS FOR VACCINE PRODUCTION | Takeda Pharmaceutical Company Limited | 1-
Sep- 16 |
1-
Sep -17 |
27-
Jun- 19 |
| US20190194
322 |
IDENTIFICATION OF VSIG3/VISTA AS A NOVEL IMMUNE CHECKPOINT AND USE THEREOF FOR IMMUNOTHERAPY | BIO-TECHNE CORPORATION | 3-
Aug- 16 |
3-
Aug -17 |
27-
Jun- 19 |
| US20190194
299 |
MIDDLE EAST RESPIRATORY SYNDROME CORONAVIRUS NEUTRALIZING ANTIBODIES AND METHODS OF USE THEREOF | Not Available | 25-
Apr- 14 |
19-
Nov -18 |
27-
Jun- 19 |
| US20190194
226 |
FUSED [1,2]IMIDAZO[4,5-C] RING COMPOUNDS SUBSTITUTED WITH GUANIDINO GROUPS | Not Available | 26-
Aug- 16 |
1-
Aug -17 |
27-
Jun- 19 |
| US20190194
150 |
COMPOUNDS AND METHODS FOR MODULATING RNA FUNCTION | Arrakis Therapeutics, Inc. | 1-Jul-
16 |
30-
Jun -17 |
27-
Jun- 19 |
| US20190192
691 |
REGULATED BIOCIRCUIT SYSTEMS | Not Available | 11-
Apr- 16 |
11-
Apr -17 |
27-
Jun- 19 |
| US20190192
581 |
Methods of Populating a Gastrointestinal Tract | Not Available | 4-
Feb- 13 |
1-
Aug -18 |
27-
Jun- 19 |
| US20190187
151 |
ASSAY FOR QUANTITATION OF PROTEINS AND PEPTIDES USING STABLE ISOTOPE STANDARDS | UVic Industry Partnerships Inc. | 6-
Jun- 16 |
7-
Apr -17 |
20-
Jun- 19 |
| US20190187
130 |
COLORS FOR CHROMOGENIC IHC AND ISH STAINING WITH MULTIDYE QUINONE METHIDE AND TYRAMIDE CONJUGATES | Not Available | 28-
Jun- 16 |
19-
Dec -18 |
20-
Jun- 19 |
| US20190185
922 |
LUMINOPHORE-LABELED MOLECULES COUPLED WITH PARTICLES FOR MICROARRAY-BASED ASSAYS | CapitalBio Corporation | 5-
Dec- 13 |
20-
Nov -18 |
20-
Jun- 19 |
| US20190185
832 |
DIET CONTROLLED EXPRESSION OF A NUCLEIC ACID ENCODING CAS9 NUCLEASE AND USES THEREOF | Not Available | 3-
Jun- 16 |
2-
Jun -17 |
20-
Jun- 19 |
| US20190184
067 |
MODIFIED ALGINATES FOR ANTI-FIBROTIC MATERIALS AND APPLICATIONS | Not Available | 1-
Nov- 15 |
28-
Feb -19 |
20-
Jun- 19 |
| US20190184
018 |
CARBOHYDRATE CONJUGATES AS DELIVERY AGENTS FOR OLIGONUCLEOTIDES | Not Available | 4-
Dec- 07 |
20-
Nov -18 |
20-
Jun- 19 |
| US20190183
968 |
METHODS AND REAGENTS FOR EFFICIENT AND TARGETED DELIVERY OF THERAPEUTIC MOLECULES TO CXCR4 CELLS | Not Available | 13-
Jan- 11 |
27-
Feb -19 |
20-
Jun- 19 |
| US20190183
918 |
SYSTEMIC IN VIVO DELIVERY OF OLIGONUCLEOTIDES | Not Available | 12-
Jun- 13 |
29-
Oct -18 |
20-
Jun- 19 |
| US20190177
739 |
RECOMBINANT INFLUENZA VIRUS-LIKE PARTICLES (VLPS) PRODUCED IN TRANSGENIC PLANTS | Not Available | 21-
Jan- 08 |
13-
Dec -18 |
13-
Jun- 19 |
| US20190175
716 |
Adenoviral Vector | Not Available | 23-
Jun- 16 |
23-
Jun -17 |
13-
Jun- 19 |
| US20190175
528 |
INHIBITION OF BIOFILM ORGANISMS | Not Available | 31-
Mar- 09 |
13-
Feb -19 |
13-
Jun- 19 |
| US20190169
677 |
PORTABLE MOLECULAR DIAGNOSTIC DEVICE AND METHODS FOR THE DETECTION OF TARGET VIRUSES | Click Diagnostics, Inc. | 9-
Nov- 17 |
9-
Nov -18 |
6-
Jun- 19 |
| US20190169
639 |
CRISPR-RELATED METHODS AND COMPOSITIONS WITH GOVERNING gRNAS | EDITAS MEDICINE, INC. | 7-
Nov- 13 |
24-
Jan -19 |
6-
Jun- 19 |
| US20190169
595 |
RNA TARGETING METHODS AND COMPOSITIONS | Salk Institute for Biological Studies | 22-
Aug- 17 |
25-
Jan -19 |
6-
Jun- 19 |
| US20190167
787 |
Methods and Compositions for Inhibiting Akt3 | Not Available | 15-
Jan- 16 |
6-
Feb -19 |
6-
Jun- 19 |
| US20190167
786 |
EMULSIONS WITH FREE AQUEOUS-PHASE SURFACTANT FOR ADJUVANTING SPLIT INFLUENZA VACCINES | Not Available | 4-
Nov- 05 |
9-
Jul- 18 |
6-
Jun- 19 |
| US20190167
636 |
METHODS FOR TREATING PULMONARY EMPHYSEMA USING SUBSTITUTED 2-AZA-BICYCLO[2.2.1]HEPTANE-3-CARBOXYLIC ACID (BENZYL-CYANO-METHYL)-AMIDES INHIBITORS OF CATHEPSIN C | Not Available | 14-
Mar- 13 |
8-
Feb -19 |
6-
Jun- 19 |
| US20190166
866 |
METHOD FOR PRODUCING A PROTEIN PHOSPHOLIPID COMPLEX FROM A CRUSTACEAN CATCH | Not Available | 4-
Dec- 17 |
30-
Nov -18 |
6-
Jun- 19 |
| US20190160
129 |
Novel Polygonum Cuspidatum Extracts and Their Use as Photodynamic Inactivating Agents | Not Available | 1-
Oct- 15 |
1-
Feb -19 |
30-
May -19 |
| US20190160
063 |
NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 31-
Dec- 15 |
30-
Dec -16 |
30-
May -19 |
| US20190154
687 |
DETECTION DEVICE AND DETECTION METHOD | Kabushiki Kaisha Toshiba | 17-
Nov- 17 |
7-
Mar -18 |
23-
May -19 |
| US20190154
550 |
BIOAEROSOL DETECTION SYSTEMS AND METHODS OF USE | Not Available | 6-
Apr- 16 |
6-
Apr -17 |
23-
May -19 |
| US20190153
471 |
COMPOSITIONS FOR THE TREATMENT OF DISEASE | Not Available | 29-
Apr- 16 |
28-
Apr -17 |
23-
May -19 |
| US20190153
086 |
Heterodimeric Immunoglobulins | Not Available | 21-
Nov- 12 |
5-
Feb -19 |
23-
May -19 |
| US20190151
844 |
DEVICES AND METHODS FOR THE DETECTION OF MOLECULES USING A FLOW CELL | Click Diagnostics, Inc. | 29-
Jun- 16 |
21-
Dec -18 |
23-
May -19 |
| US20190151
474 |
RNA-BASED LOGIC CIRCUITS WITH RNA BINDING PROTEINS, APTAMERS AND SMALL MOLECULES | Kyoto University | 8-
Sep- 14 |
8-
Sep -15 |
23-
May -19 |
| US20190144
930 |
ENHANCED METHODS OF RIBONUCLEIC ACID HYBRIDIZATION | Not Available | 6-
Dec- 13 |
9-
Jan -19 |
16-
May -19 |
| US20190144
929 |
DEVICES FOR CRISPR EFFECTOR SYSTEM BASED DIAGNOSTICS | Not Available | 15-
Mar- 17 |
15-
Mar -18 |
16-
May -19 |
| US20190144
827 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | RUBIUS THERAPEUTICS, INC. | 18-
Nov- 13 |
19-
Nov -18 |
16-
May -19 |
| US20190144
556 |
ANTAGONISTIC ANTI-TUMOR NECROSIS FACTOR RECEPTOR SUPERFAMILY ANTIBODIES | Not Available | 13-
May- 16 |
12-
May -17 |
16-
May -19 |
| US20190144
484 |
ALKYNE CONTAINING NUCLEOTIDE AND NUCLEOSIDE THERAPEUTIC COMPOSITIONS AND USES RELATED THERETO | Not Available | 28-
Apr- 16 |
28-
Apr -17 |
16-
May -19 |
| US20190142
929 |
INFLUENZA VACCINE REGIMENS FOR PANDEMIC ASSOCIATED STRAINS | Not Available | 10-
Feb- 09 |
15-
Oct -18 |
16-
May -19 |
| US20190142
927 |
LOW-ADDITIVE INFLUENZA VACCINES | Not Available | 27-
Jun- 07 |
26-
Jun -18 |
16-
May -19 |
| US20190136
226 |
DEVICES AND METHODS FOR NUCLEIC ACID EXTRACTION | Not Available | 11-
May- 16 |
9-
Nov -18 |
9-
May -19 |
| US20190136
215 |
Replication Conditional Virus that Specifically Kills Senescent Cells | Not Available | 17-
Apr- 12 |
6-
Jul- 18 |
9-
May -19 |
| US20190135
875 |
TRI-SEGMENTED PICHINDE VIRUSES AS VACCINE VECTORS | Not Available | 18-
May- 16 |
17-
May -17 |
9-
May -19 |
| US20190135
873 |
COMPOSITIONS AND METHODS FOR TREATING DISEASES BY INHIBITING EXOSOME RELEASE | Not Available | 19-
Dec- 16 |
19-
Dec -18 |
9-
May -19 |
| US20190135
788 |
DIHYDROPYRIMIDINYL BENZAZEPINE CARBOXAMIDE COMPOUNDS | Hoffmann-La Roche Inc. | 12-
Jun- 16 |
7-
Dec -18 |
9-
May -19 |
| US20190134
214 |
GLP-1 Receptor Ligand Moiety Conjugated Oligonucleotides and Uses Thereof | Not Available | 6-
May- 16 |
4-
May -17 |
9-
May -19 |
| US20190134
193 |
USE OF TAM RECEPTOR INHIBITORS AS IMMUNOENHANCERS AND TAM ACTIVATORS AS IMMUNOSUPPRESSORS | SALK INSTITUTE FOR BIOLOGICAL STUDIES | 9-
Nov- 07 |
9-
Aug -18 |
9-
May -19 |
| US20190134
186 |
INFLUENZA VACCINES WITH REDUCED AMOUNT OF EMULSION ADJUVANT | Not Available | 4-
Nov- 05 |
10-
Jul- 18 |
9-
May -19 |
| US20190134
062 |
PHARMACEUTICAL COMPOSITIONS AND METHODS | Not Available | 3-
Aug- 15 |
8-
Jan -19 |
9-
May -19 |
| US20190128
893 |
QUINONE METHIDE ANALOG SIGNAL AMPLIFICATION | Not Available | 24-
Feb- 14 |
7-
Nov -18 |
2-
May -19 |
| US20190128
810 |
SYSTEM FOR MEASURING OPTICAL SIGNAL DETECTOR PERFORMANCE | Not Available | 31-
Dec- 15 |
13-
Dec -18 |
2-
May -19 |
| US20190127
441 |
METHODS AND COMPOSITIONS FOR REDUCING VIRUS INFECTIVITY | University of Vermont and State Agricultural College | 13-
Apr- 16 |
13-
Apr -17 |
2-
May -19 |
| US20190127
405 |
QUINONE METHIDE ANALOG SIGNAL AMPLIFICATION | Not Available | (Ml)
LL |
7-
Nov -18 |
2-
May -19 |
| US20190127
401 |
CHARGED LINKERS AND THEIR USES FOR CONJUGATION | HANGZHOU DAC BIOTECH CO., LTD. | 12-
Aug- 16 |
20-
Dec -18 |
2-
May -19 |
| US20190127
400 |
CHARGED LINKERS AND THEIR USES FOR CONJUGATION | HANGZHOU DAC BIOTECH CO., LTD. | 12-
Aug- 16 |
20-
Dec -18 |
2-
May -19 |
| US20190127
399 |
CHARGED LINKERS AND THEIR USES FOR CONJUGATION | HANGZHOU DAC BIOTECH CO., LTD. | 12-
Aug- 16 |
20-
Dec -18 |
2-
May -19 |
| US20190125
858 |
COLD ADAPTED AND VIRULENCE FACTOR DELETED LIVE
ATTENUATED VACCINE SUITABLE FOR MUCOSAL DELIVERY |
Not Available | 18-
Apr- 16 |
18-
Apr -17 |
2-
May -19 |
| US20190125
806 |
Antimicrobial and Antiviral Agent, Antimicrobial and Antiviral Member, and Method for Producing Antimicrobial and Antiviral Agent | Murata Manufacturing Co., Ltd. | 13-
Jun- 16 |
13-
Dec -18 |
2-
May -19 |
| US20190125
724 |
PREVENTION AND TREATMENT OF VIRAL INFECTIONS | Not Available | 2-
Jun- 16 |
27-
Nov -18 |
2-
May -19 |
| US20190119
744 |
CAPTURE PRIMERS AND CAPTURE SEQUENCE LINKED SOLID SUPPORTS FOR MOLECULAR DIAGNOSTIC TESTS | Not Available | 31-
Jul-09 |
5-
Nov -18 |
25-
Apr- 19 |
| US20190119
743 |
COMPOSITIONS AND METHODS FOR DETECTING RARE SEQUENCE VARIANTS | Not Available | 15-
Aug- 16 |
26-
Oct -18 |
25-
Apr- 19 |
| US20190119
701 |
METHODS FOR IMPROVED HOMOLOGOUS RECOMBINATION AND COMPOSITIONS THEREOF | Not Available | 8-
Sep- 17 |
7-
Sep -18 |
25-
Apr- 19 |
| US20190119
266 |
COMPOSITIONS AND METHODS FOR INHIBITING KINASES | Not Available | 23-
Apr- 15 |
24-
Oct -18 |
25-
Apr- 19 |
| US20190119
220 |
CARBOXYLIC ACID COMPOUNDS | Sumitomo Dainippon Pharma Co., Ltd. | 18-
May- 12 |
23-
Oct -18 |
25-
Apr- 19 |
| US20190117
793 |
MODULAR NANODEVICES FOR SMART ADAPTABLE VACCINES | Not Available | 15-
Feb- 07 |
1-
Oct -18 |
25-
Apr- 19 |
| US20190117
702 |
USE OF ASC AND ASC-CM TO TREAT ARDS, SARS, AND MERS | Indiana University Research and Technology Corporation | 6-
May- 14 |
31-
Oct -18 |
25-
Apr- 19 |
| US20190112
596 |
TAL-EFFECTOR ASSEMBLY PLATFORM, CUSTOMIZED SERVICES, KITS AND ASSAYS | Not Available | 4-
Apr- 12 |
12-
Apr -18 |
18-
Apr- 19 |
| US20190112
394 |
USING SORTASES TO INSTALL CLICK CHEMISTRY HANDLES FOR PROTEIN LIGATION | Whitehead Institute for Biomedical Research | 28-
Jun- 11 |
24-
Sep -18 |
18-
Apr- 19 |
| US20190111
141 |
A PEPTIDE WITH ABILITY TO PENETRATE CELL MEMBRANE | Ewha University – Industry Collaboration Foundation | 6-
Apr- 16 |
6-
Apr -17 |
18-
Apr- 19 |
| US20190105
653 |
PORTABLE PATHOGEN ANALYSIS SYSTEM FOR DETECTING WATERBORNE PATHOGENS | Not Available | 5-
Oct- 17 |
5-
Oct -18 |
11-
Apr- 19 |
| US20190105
381 |
METHOD FOR PREPARING VIRAL PARTICLES WITH CYCLIC DINUCLEOTIDE AND USE OF SAID PARTICLES FOR TREATING CANCER | Not Available | 16-
Mar- 16 |
16-
Mar -16 |
11-
Apr- 19 |
| US20190105
334 |
Anti-Viral Azide Containing Compounds | Not Available | 28-
Jul-10 |
3-
Dec -18 |
11-
Apr- 19 |
| US20190100
586 |
Humanized Anti-Claudin-1 Antibodies and Uses Thereof | Chu Strasbourg, Les HA’pitaux Universitaires de Strasbourg | 22-
Mar- 16 |
21-
Mar -17 |
4-
Apr- 19 |
| US20190099
493 |
Targeting Lipids | Arbutus Biopharma Corporation | 4-
Dec- 07 |
10-
Oct -17 |
4-
Apr- 19 |
| US20190099
479 |
RECOMBINANT HCMV AND RHCMV VECTORS AND USES THEREOF | Not Available | 14-
May- 10 |
27-
Apr -18 |
4-
Apr- 19 |
| US20190094
224 |
METHODS AND COMPOSITIONS FOR DETECTING SINGLE T CELL RECEPTOR AFFINITY AND SEQUENCE | Not Available | 11-
Apr- 16 |
6-
Apr -17 |
28-
Mar -19 |
| US20190091
329 |
CATIONIC OIL-IN-WATER EMULSIONS | GLAXOSMITHKLINE BIOLOGICALS, SA | 6-Jul-
11 |
6-
Dec -18 |
28-
Mar -19 |
| US20190091
221 |
METHODS AND COMPOSITIONS FOR TREATING VIRAL OR VIRALLY- INDUCED CONDITIONS | TRUSTEES OF BOSTON UNIVERSITY | 11-
Mar- 10 |
23-
Apr -18 |
28-
Mar -19 |
| US20190085
057 |
MAST CELL STABILIZERS FOR TREATMENT OF HYPERCYTOKINEMIA AND VIRAL INFECTION | Not Available | 8-
Sep- 16 |
15-
Nov -18 |
21-
Mar -19 |
| US20190085
024 |
Alpha-Ketoamide Inhibitors Of Cysteine Proteases | Not Available | 15-
Sep- 17 |
17-
Sep -18 |
21-
Mar -19 |
| US20190085
013 |
NUCLEOTIDE AND NUCLEOSIDE THERAPEUTIC COMPOSITIONS AND USES RELATED THERETO | Not Available | 7-
Mar- 16 |
7-
Mar -17 |
21-
Mar -19 |
| US20190084
943 |
CHLORO-PYRAZINE CARBOXAMIDE DERIVATIVES WITH EPITHELIAL
SODIUM CHANNEL BLOCKING ACTIVITY |
Parion Sciences, Inc. | 17-
Dec- 12 |
23-
Aug -18 |
21-
Mar -19 |
| US20190083
602 |
METHOD FOR PRODUCING RNA MOLECULE COMPOSITIONS | Not Available | 22-
Dec- 15 |
22-
Dec -16 |
21-
Mar -19 |
| US20190083
592 |
IMMUNOSTIMULATORY COMBINATIONS | Not Available | 30-
Dec- 02 |
22-
Oct -18 |
21-
Mar -19 |
| US20190083
569 |
MICROBICIDAL COMPOSITIONS AND METHODS FOR TREATMENT OF VIRAL INFECTIONS | Not Available | 11-
Jun- 15 |
24-
Jul- 18 |
21-
Mar -19 |
| US20190083
525 |
COMPOSITIONS COMPRISING AN RNA POLYMERASE INHIBITOR AND CYCLODEXTRIN FOR TREATING VIRAL INFECTIONS | Not Available | 11-
Jul-17 |
10-
Jul- 18 |
21-
Mar -19 |
| US20190083
520 |
N4-Hydroxycytidine and Derivatives and Anti-Viral Uses Related Thereto | Not Available | 10-
Mar- 16 |
10-
Mar -17 |
21-
Mar -19 |
| US20190083
408 |
CONTROLLED-RELEASE PEPTIDE COMPOSITIONS AND USES THEREOF | Not Available | 2-
Dec- 11 |
4-
Dec -18 |
21-
Mar -19 |
| US20190083
397 |
OIL-IN-WATER EMULSIONS INCLUDING RETINOIC ACID | NOVARTIS AG | 23-
Dec- 15 |
21-
Dec -16 |
21-
Mar -19 |
| US20190078
060 |
DECREASING POTENTIAL IATROGENIC RISKS ASSOCIATED WITH INFLUENZA VACCINES | Novartis AG | 9-
Sep- 04 |
8-
Nov -18 |
14-
Mar -19 |
| US20190078
051 |
Animal Protein-Free Media for Cultivation of Cells | Baxalta GmbH | 29-
Oct- 04 |
29-
Oct -18 |
14-
Mar -19 |
| US20190077
847 |
AMINO ACID SEQUENCES DIRECTED AGAINST ENVELOPE PROTEINS OF A VIRUS AND POLYPEPTIDES COMPRISING THE SAME FOR THE TREATMENT OF VIRAL DISEASES | Ablynx N.V. | 5-
Jun- 08 |
17-
Oct -17 |
14-
Mar -19 |
| US20190077
764 |
BENZAZEPINE DICARBOXAMIDE COMPOUNDS WITH SECONDARY AMIDE FUNCTION | Hoffmann-La Roche Inc. | 23-
May- 16 |
13-
Nov -18 |
14-
Mar -19 |
| US20190077
763 |
BENZAZEPINE DICARBOXAMIDE COMPOUNDS WITH TERTIARY AMIDE FUNCTION | Hoffmann-La Roche Inc. | 23-
May- 16 |
13-
Nov -18 |
14-
Mar -19 |
| US20190076
520 |
VACCINES AND IMMUNOTHERAPEUTICS USING IL-28 AND COMPOSITIONS AND METHODS OF USING | Not Available | 4-
Apr- 08 |
11-
Sep -18 |
14-
Mar -19 |
| US20190076
468 |
ENHANCED IMMUNE RESPONSE UPON TREATMENT WITH NITRIC OXIDE | Not Available | 11-
Sep- 17 |
11-
Sep -17 |
14-
Mar -19 |
| US20190071
423 |
QUINAZOLINONES AND AZAQUINAZOLINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
2-
Nov -18 |
7-
Mar -19 |
| US20190071
422 |
QUINAZOLINONES AND AZAQUINAZOLINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
2-
Nov -18 |
7-
Mar -19 |
| US20190071
421 |
QUINAZOLINONES AND AZAQUINAZOLINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
2-
Nov -18 |
7-
Mar -19 |
| US20190071
420 |
QUINAZOLINONES AND AZAQUINAZOLINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
2-
Nov -18 |
7-
Mar -19 |
| US20190071
419 |
QUINAZOLINONES AND AZAQUINAZOLINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
2-
Nov -18 |
7-
Mar -19 |
| US20190071
418 |
QUINAZOLINONES AND AZAQUINAZOLINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
2-
Nov -18 |
7-
Mar -19 |
| US20190062
785 |
MULTIVALENT VACCINES FOR RABIES VIRUS AND CORONOVIRUSES | Not Available | 4-
Apr- 16 |
31-
Mar -17 |
28-
Feb -19 |
| US20190062
724 |
RNA TARGETING METHODS AND COMPOSITIONS | Salk Institute for Biological Studies | 22-
Aug- 17 |
27-
Mar -18 |
28-
Feb -19 |
| US20190062
713 |
HIGHLY EFFICIENT INFLUENZA MATRIX (M1) PROTEINS | Not Available | 11-
Jul-03 |
22-
Mar -18 |
28-
Feb -19 |
| US20190062
408 |
CONSERVED HEMAGGLUTININ EPITOPE, ANTIBODIES TO THE EPITOPE, AND METHODS OF USE | Not Available | 25-
Aug- 08 |
20-
Jun -18 |
28-
Feb -19 |
| US20190062
380 |
FUSION PROTEINS FOR PROMOTING AN IMMUNE RESPONSE, NUCLEIC ACIDS ENCODING SAME, AND METHODS OF MAKING AND USE THEREOF | Not Available | 7-
Sep- 12 |
28-
Aug -18 |
28-
Feb -19 |
| US20190062
326 |
2-PHENYL-3-(PIPERAZINOMETHYL)IMIDAZO[1,2-A] PYRIDINE DERIVATIVES AS BLOCKERS OF TASK-1 AND TASK-2 CHANNELS, FOR THE TREATMENT OF SLEEP-RELATED BREATHING DISORDERS | BAYER PHARMA AKTIENGESELLSCHAFT | 10-
Dec- 15 |
7-
Dec -16 |
28-
Feb -19 |
| US20190062
323 |
PI-Kinase Inhibitors with Anti-Infective Activity | Not Available | 26-
Feb- 16 |
24-
Feb -17 |
28-
Feb -19 |
| US20190060
435 |
PEPTIDE VACCINE FORMULATIONS AND USE THEREOF FOR INDUCING AN IMMUNE RESPONSE | The United States of America, as represented by the Secretary, Dept of Health and Human Service | 27-
Feb- 16 |
27-
Feb -17 |
28-
Feb -19 |
| US20190060
364 |
INTRACELLULAR GENOMIC TRANSPLANT AND METHODS OF THERAPY | Not Available | 31-
Jul-15 |
6-
Nov -18 |
28-
Feb -19 |
| US20190060
363 |
INTRACELLULAR GENOMIC TRANSPLANT AND METHODS OF THERAPY | Not Available | 31-
Jul-15 |
6-
Nov -18 |
28-
Feb -19 |
| US20190060
262 |
ENHANCED EXPRESSION OF RNA VECTORS | UNIVERSITY COURT OF THE UNIVERSITY OF EDINBURGH | 25-
Mar- 13 |
5-
Sep -18 |
28-
Feb -19 |
| US20190060
239 |
Technology for the Preparation of Microparticles | Not Available | 24-
Jul-07 |
17-
Oct -18 |
28-
Feb -19 |
| US20190056
122 |
Clean Rooms Having Dilute Hydrogen Peroxide (DHP) Gas and Methods of Use Thereof | Synexis LLC | 20-
Apr- 15 |
20-
Apr -16 |
21-
Feb -19 |
| US20190055
256 |
ANTI-VIRAL DRUG | DORING INTERNATIONAL GMBH | 24-
Feb- 16 |
24-
Feb -17 |
21-
Feb -19 |
| US20190055
241 |
GUANIDINE SUBSTITUTED IMIDAZO[4,5-c] RING COMPOUNDS | Not Available | 31-
Aug- 15 |
22-
Oct -18 |
21-
Feb -19 |
| US20190055
234 |
COMPOSITIONS AND METHODS FOR INHIBITING KINASES | Not Available | 23-
Apr- 15 |
24-
Oct -18 |
21-
Feb -19 |
| US20190054
188 |
ADENO-ASSOCIATED VIRUS (AAV) CLADES, SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 30-
Sep- 03 |
2-
Oct -18 |
21-
Feb -19 |
| US20190054
127 |
ANTIVIRAL AGENT AND ANTIVIRAL FOOD | EDUCATIONAL CORPORATION MUKOGAWA GAKUIN | 4-
Mar- 16 |
22-
Nov -16 |
21-
Feb -19 |
| US20190054
122 |
INTRACELLULAR GENOMIC TRANSPLANT AND METHODS OF THERAPY | Not Available | 31-
Jul-15 |
5-
Nov -18 |
21-
Feb -19 |
| US20190049
378 |
CONTINUOUS PROCESS FOR PERFORMING MULTIPLE NUCLEIC ACID AMPLIFICATION ASSAYS | Not Available | 10-
Mar- 05 |
21-
Jun -18 |
14-
Feb -19 |
| US20190048
344 |
CHEMICAL MODIFICATIONS OF MONOMERS AND OLIGONUCLEOTIDES WITH CYCLOADDITION | Not Available | 23-
Sep- 08 |
16-
Aug -18 |
14-
Feb -19 |
| US20190048
082 |
PSEUDOTYPED ONCOLYTIC VIRAL DELIVERY OF THERAPEUTIC POLYPEPTIDES | Not Available | 30-
Jun- 16 |
25-
Oct -18 |
14-
Feb -19 |
| US20190048
049 |
CARGOMERS | CERENIS THERAPEUTICS HOLDING SA | 10-
Aug- 17 |
10-
Aug -18 |
14-
Feb -19 |
| US20190048
026 |
Boron-Containing Small Molecules | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
18-
Oct -18 |
14-
Feb -19 |
| US20190046
690 |
MATERIALS WITH IMPROVED PROPERTIES | Not Available | 1-
Nov- 15 |
2-
Nov -16 |
14-
Feb -19 |
| US20190046
654 |
ALBUMIN BINDING PEPTIDE CONJUGATES AND METHODS THEREOF | Not Available | 9-
Aug- 17 |
9-
Aug -18 |
14-
Feb -19 |
| US20190046
635 |
COMPOSITION FOR IMMUNITY INDUCTION PROMOTION AND VACCINE PHARMACEUTICAL COMPOSITION | NITTO DENKO CORPORATION | 2-
Feb- 16 |
1-
Feb -17 |
14-
Feb -19 |
| US20190040
451 |
FULLY INTEGRATED HAND-HELD DEVICE TO DETECT SPECIFIC NUCLEIC ACID SEQUENCES | Not Available | 8-
Jan- 16 |
9-
Jan -17 |
7-
Feb -19 |
| US20190040
378 |
NOVEL NUCLEIC ACID MOLECULES | Not Available | 4-Jul-
17 |
3-
Jul- 18 |
7-
Feb -19 |
| US20190040
370 |
TRACKING AND MANIPULATING CELLULAR RNA VIA NUCLEAR DELIVERY OF CRISPR/CAS9 | Not Available | 23-
Nov- 15 |
3-
Aug -18 |
7-
Feb -19 |
| US20190040
105 |
Method for Preventing and Treating Hyperpermeability | Not Available | 5-
Mar- 09 |
19-
Oct -18 |
7-
Feb -19 |
| US20190038
742 |
MESENCHYMAL STEM CELLS AS VACCINE ADJUVANTS AND METHODS FOR USING THE SAME | Longeveron LLC | 4-
Feb- 16 |
2-
Feb -17 |
7-
Feb -19 |
| US20190032
077 |
ARTIFICIAL NUCLEIC ACID MOLECULES | CureVac AG | 30-
Dec- 13 |
9-
Jul- 18 |
31-
Jan- 19 |
| US20190032
041 |
COMPOSITIONS FOR AND METHODS OF IDENTIFYING ANTIGENS | Not Available | 21-
Feb- 06 |
12-
Feb -18 |
31-
Jan- 19 |
| US20190031
740 |
COMPOSITIONS COMPRISING AAV EXPRESSING DUAL ANTIBODY CONSTRUCTS AND USES THEREOF | Not Available | 13-
May- 14 |
15-
Oct -18 |
31-
Jan- 19 |
| US20190031
679 |
NOVEL MONOTHIOL MUCOLYTIC AGENTS | Not Available | 30-
Jan- 15 |
5-
Sep -18 |
31-
Jan- 19 |
| US20190031
605 |
TETRAHYDRONAPHTHALENE DERIVATIVE | ONO PHARMACEUTICAL CO., LTD. | 29-
Jan- 16 |
27-
Jan -17 |
31-
Jan- 19 |
| US20190030
187 |
sirna/Nanoparticle Formulations for Treatment of Middle-East Respiratory Syndrome Coronaviral Infection | Sirnaomics, Inc. | 8-
Sep- 15 |
7-
Sep -16 |
31-
Jan- 19 |
| US20190030
094 |
BACTERIAL STRAIN AS AGENTS FOR PREVENTING AND/OR TREATING RESPIRATORY DISORDERS | Not Available | 27-
Jan- 16 |
27-
Jan -17 |
31-
Jan- 19 |
| US20190025
292 |
ANTIGEN PRESENTING CELL ASSAY | University of Pittsburgh – Of the Com monwealth System of Higher Education | 8-
Apr- 10 |
25-
Sep -18 |
24-
Jan- 19 |
| US20190024
096 |
PROCESS FOR THE IN VIVO PRODUCTION OF RNA IN A HOST CELL | Not Available | 7-
Aug- 15 |
7-
Aug -15 |
24-
Jan- 19 |
| US20190023
799 |
GITR Antibodies And Methods Of Inducing Or Enhancing An Immune Response | Not Available | 25-
Mar- 05 |
28-
Jun -18 |
24-
Jan- 19 |
| US20190023
779 |
METHOD OF PROVIDING MONOCLONAL AUTO-ANTIBODIES WITH DESIRED SPECIFICITY | Not Available | 28-
Dec- 11 |
5-
Oct -18 |
24-
Jan- 19 |
| US20190023
769 |
COMPOSITIONS AND METHODS FOR INHIBITING PATHOGEN INFECTION | Not Available | 29-
Oct- 12 |
21-
Sep -18 |
24-
Jan- 19 |
| US20190022
249 |
ADENO-ASSOCIATED VIRUS (AAV) CLADES, SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 30-
Sep- 03 |
2-
Oct -18 |
24-
Jan- 19 |
| US20190022
216 |
ANTIBODY/T-CELL RECEPTOR CHIMERIC CONSTRUCTS AND USES THEREOF | Not Available | 23-
Oct- 15 |
4-
Sep -18 |
24-
Jan- 19 |
| US20190022
214 |
Attenuated Infectious Bronchitis Virus | Not Available | 27-
Jan- 16 |
26-
Jan -17 |
24-
Jan- 19 |
| US20190022
213 |
MERS-CoV Vaccine | Not Available | 29-
Nov- 13 |
29-
Jun -18 |
24-
Jan- 19 |
| US20190022
116 |
N4-Hydroxycytidine and Derivatives and Anti-Viral Uses Related Thereto | Not Available | 26-
Dec- 14 |
16-
Dec -15 |
24-
Jan- 19 |
| US20190017
112 |
METHOD OF DIRECT TARGET SEQUENCING USING NUCLEASE PROTECTION | HTG Molecular Diagnostics, Inc. | 11-
Feb- 16 |
10-
Feb -17 |
17-
Jan- 19 |
| US20190017
068 |
ADENO-ASSOCIATED VIRUS (AAV) SEROTYPE 8 SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 17-
Dec- 01 |
26-
Sep -18 |
17-
Jan- 19 |
| US20190017
000 |
CLEANING COMPOSITION, METHOD OF MAKING AND USE THEREOF | Not Available | 8-
Sep- 16 |
19-
Sep -18 |
17-
Jan- 19 |
| US20190016
785 |
ANTIBODIES THAT POTENTLY NEUTRALIZE HEPATITIS B VIRUS
AND USES THEREOF |
Not Available | 7-
Oct- 15 |
7-
Oct -16 |
17-
Jan- 19 |
| US20190016
772 |
GM-CSF and IL-4 Conjugates, Compositions, and Methods Related Thereto | Not Available | 23-
Oct- 12 |
2-
Oct -18 |
17-
Jan- 19 |
| US20190016
710 |
MULTICYCLIC COMPOUNDS AND USES THEREOF | Not Available | 31-
Dec- 15 |
29-
Dec -16 |
17-
Jan- 19 |
| US20190016
690 |
NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 31-
Dec- 15 |
30-
Dec -16 |
17-
Jan- 19 |
| US20190015
527 |
ADENO-ASSOCIATED VIRUS (AAV) CLADES, SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 30-
Sep- 03 |
25-
Jul- 18 |
17-
Jan- 19 |
| US20190015
522 |
IMMUNOSTIMULATORY COMPOSITIONS AND METHODS OF USE THEREOF | Not Available | 5-
Apr- 12 |
19-
Jul- 18 |
17-
Jan- 19 |
| US20190015
501 |
NUCLEIC ACID VACCINES | ModernaTX, Inc. | 23-
Apr- 14 |
27-
Sep -18 |
17-
Jan- 19 |
| US20190015
432 |
Lipid Disulfide Prodrugs and Uses Related Thereto | Not Available | 13-
Jul-17 |
13-
Jul- 18 |
17-
Jan- 19 |
| US20190010
469 |
ATTENUATED VIRUSES USEFUL FOR VACCINES | Not Available | 30-
Mar- 07 |
16-
Jul- 18 |
10-
Jan- 19 |
| US20190010
240 |
COMPOSITION COMPRISED OF ANTIGEN LINKED TO A TNF SUPERFAMILY LIGAND | Not Available | 15-
Mar- 13 |
2-
Aug -18 |
10-
Jan- 19 |
| US20190010
132 |
ARYLALKYL-AND ARYLOXYALKYL-SUBSTITUTED EPITHELIAL SODIUM CHANNEL BLOCKING COMPOUNDS | Parion Sciences, Inc. | 13-
Dec- 13 |
4-
Apr -18 |
10-
Jan- 19 |
| US20190008
954 |
NEGATIVELY CHARGED NUCLEIC ACID COMPRISING COMPLEXES FOR IMMUNOSTIMULATION | CureVac AG | 31-
Jan- 12 |
11-
Jun -18 |
10-
Jan- 19 |
| US20190008
948 |
NUCLEIC ACID VACCINES | ModernaTX, Inc. | 23-
Apr- 14 |
16-
Jul- 18 |
10-
Jan- 19 |
| US20190008
833 |
NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 9-
May- 12 |
17-
Jul- 18 |
10-
Jan- 19 |
| US20190004
061 |
DETECTING TARGETS USING MASS TAGS AND MASS SPECTROMETRY | Not Available | 2-Jul-
10 |
4-
Aug -18 |
3-
Jan- 19 |
| US20190002
477 |
ANTI-VIRAL COMPOUNDS, PHARMACEUTICAL COMPOSITIONS, AND METHODS OF USE THEREOF | Kineta, Inc. | 9-
May- 14 |
31-
Jan -18 |
3-
Jan- 19 |
| US20190002
448 |
SUBSTITUTED BENZOFURANYL AND BENZOXAZOLYL COMPOUNDS AND USES THEREOF | Not Available | 31-
Dec- 15 |
29-
Dec -16 |
3-
Jan- 19 |
| US20190002
393 |
Lipids and Lipid Compositions for the Delivery of Active Agents | Not Available | 5-
Sep- 14 |
19-
Sep -18 |
3-
Jan- 19 |
| US20190001
010 |
ADDITIVE COMPOSITIONS FOR PIGMENTED DISINFECTION AND METHODS THEREOF | Not Available | 8-
Dec- 14 |
13-
Jul- 18 |
3-
Jan- 19 |
| US20190000
959 |
NUCLEIC ACID VACCINES | ModernaTX, Inc. | 23-
Apr- 14 |
27-
Jul- 18 |
3-
Jan- 19 |
| US20190000
745 |
POLYMER-BASED ANTIMICROBIAL COMPOSITIONS AND METHODS OF USE THEREOF | eXion labs Inc. | 28-
Jul-16 |
4-
Sep -18 |
3-
Jan- 19 |
| US20180372
747 |
METHODS OF IDENTIFYING IMMUNE CELLS IN PD-L1 POSITIVE TUMOR TISSUE | Not Available | 22-
Nov- 15 |
21-
May -18 |
27-
Dec -18 |
| US20180372
733 |
ANTIBODY-NANOPARTICLE CONJUGATES AND METHODS FOR MAKING AND USING SUCH CONJUGATES | Not Available | 27-
Apr- 10 |
21-
Jun -18 |
27-
Dec -18 |
| US20180371
536 |
METHODS FOR RNA QUANTIFICATION | Not Available | 1-
Jun- 15 |
26-
May -16 |
27-
Dec -18 |
| US20180371
461 |
APTAMERS, NUCLEIC ACID MOLECULES, POLYNUCLEOTIDES, SYNTHETIC ANTIBODIES COMPOSITIONS FOR DETECTING PRRS VIRUSES AND TREATING PRRS VIRUS INFECTION | Not Available | 10-
Dec- 15 |
1-
Dec -16 |
27-
Dec -18 |
| US20180371
410 |
MEANS AND METHODS FOR INFLUENCING THE STABILITY OF CELLS | Not Available | 4-
Dec- 09 |
28-
Aug -18 |
27-
Dec -18 |
| US20180369
386 |
LIPIDS AND LIPID COMPOSITIONS FOR THE DELIVERY OF ACTIVE AGENTS | Not Available | 8-
Mar- 13 |
29-
Aug -18 |
27-
Dec -18 |
| US20180369
364 |
RECOMBINANT VIRUS LIKE PARTICLES USING BOVINE IMMUNODEFICIENCY VIRUS GAG PROTEIN | Not Available | 2-Jul-
15 |
1-
Jul- 16 |
27-
Dec -18 |
| US20180368
417 |
ANTIMICROBIAL COMPOSITIONS AND METHODS | Not Available | 23-
Sep- 14 |
19-
Jun -18 |
27-
Dec -18 |
| US20180365
375 |
METHODS AND SYSTEMS FOR MULTIPLE TAXONOMIC CLASSIFICATION | Not Available | 24-
Apr- 15 |
4-
Oct -17 |
20-
Dec -18 |
| US20180363
027 |
STABILIZING COMPOSITIONS AND METHODS FOR EXTRACTION OF RIBONUCLEIC ACID | Not Available | 6-
Oct- 06 |
15-
May -18 |
20-
Dec -18 |
| US20180362
625 |
REGULATION OF CYTOKINE PRODUCTION | Not Available | 4-
Dec- 15 |
2-
Dec -16 |
20-
Dec -18 |
| US20180360
877 |
METHODS FOR EXPANDING A POPULATION OF ALVEOLAR MACROPHAGES IN A LONG TERM CULTURE | Not Available | 8-
Dec- 15 |
8-
Dec -16 |
20-
Dec -18 |
| US20180360
736 |
FILM DOSAGE FORM WITH EXTENDED RELEASE MUCOADHESIVE PARTICLES | Intelgenx Corp. | 2-
Dec- 13 |
23-
Aug -18 |
20-
Dec -18 |
| US20180355
017 |
COMPOSITIONS AND METHODS FOR INTERNALIZING ENZYMES | Not Available | 7-
Jun- 17 |
6-
Jun -18 |
13-
Dec -18 |
| US20180353
594 |
METHOD FOR INACTIVATING VIRUSES USING ELECTRON BEAMS | Not Available | 26-
Jul-13 |
21-
Aug -18 |
13-
Dec -18 |
| US20180346
574 |
ANTI-PD-L1 ANTIBODIES AND USES THEREOF | Not Available | 13-
Jun- 16 |
9-
Aug -18 |
6-
Dec -18 |
| US20180346
573 |
ANTI-PD-L1 ANTIBODIES AND USES THEREOF | Not Available | 13-
Jun- 16 |
9-
Aug -18 |
6-
Dec -18 |
| US20180346
522 |
HUMAN RESPIRATORY SYNCYTIAL VIRUS CONSENSUS ANTIGENS, NUCLEIC ACID CONSTRUCTS AND VACCINES MADE THEREFROM, AND METHODS OF USING THE SAME | Not Available | 10-
Apr- 12 |
6-
Aug -18 |
6-
Dec -18 |
| US20180346
516 |
PEPTIDES AND USES THEREFOR AS ANTIVIRAL AGENTS | Not Available | 27-
Nov- 15 |
28-
Nov -16 |
6-
Dec -18 |
| US20180346
485 |
ISOTHIAZOLOPYRIMIDINONES, PYRAZOLOPYRIMIDINONES, AND PYRROLOPYRIMIDINONES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
27-
Feb -18 |
6-
Dec -18 |
| US20180346
480 |
THIENOPYRIMIDINONES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
1-
Mar -18 |
6-
Dec -18 |
| US20180344
877 |
RECOMBINANT PROMOTERS AND VECTORS FOR PROTEIN EXPRESSION IN LIVER AND USE THEREOF | Children’s Healthcare of Atlanta, Inc. | 16-
Apr- 15 |
8-
Aug -18 |
6-
Dec -18 |
| US20180344
832 |
METHODS AND COMPOSITIONS FOR COMBINATION IMMUNOTHERAPY | Not Available | 20-
Apr- 15 |
20-
Apr -16 |
6-
Dec -18 |
| US20180344
751 |
Broad Spectrum Antiviral and Methods of Use | Not Available | 17-
Apr- 06 |
26-
Dec -17 |
6-
Dec -18 |
| US20180340
219 |
CRISPR EFFECTOR SYSTEM BASED DIAGNOSTICS | MASSACHUSETTS INSTITUTE OF TECHNOLOGY | 9-
Dec- 16 |
9-
Mar -18 |
29-
Nov -18 |
| US20180340
218 |
CRISPR EFFECTOR SYSTEM BASED DIAGNOSTICS | MASSACHUSETTS INSTITUTE OF TECHNOLOGY | 9-
Dec- 16 |
9-
Mar -18 |
29-
Nov -18 |
| US20180340
215 |
SAMPLE ANALYSIS, PRESENCE DETERMINATION OF A TARGET SEQUENCE | Not Available | 28-
Aug- 15 |
26-
Aug -16 |
29-
Nov -18 |
| US20180340
154 |
PRODUCTION OF VIRUSES IN AVIAN EGGS | Not Available | 24-
Nov- 15 |
23-
Nov -16 |
29-
Nov -18 |
| US20180340
153 |
PRODUCTION OF VIRUSES IN CELL CULTURE | Not Available | 24-
Nov- 15 |
23-
Nov -16 |
29-
Nov -18 |
| US20180339
991 |
PYRROLOTRIAZINONES AND IMIDAZOTRIAZINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 30-
Dec- 14 |
24-
May -18 |
29-
Nov -18 |
| US20180339
988 |
PYRROLO AND PYRAZOLOPYRIMIDINES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 30-
Dec- 14 |
18-
Jan -18 |
29-
Nov -18 |
| US20180339
014 |
PEPTIDOMIMETIC MACROCYCLES | Not Available | 14-
Jan- 09 |
7-
Jun -18 |
29-
Nov -18 |
| US20180334
480 |
CORONAVIRUSES EPITOPE-BASED VACCINES | RAMOT AT TEL-AVIV UNIVERSITY LTD. | 17-
Sep- 15 |
15-
Sep -16 |
22-
Nov -18 |
| US20180333
485 |
Compositions, Comprising Improved Il-12 Genetic Constructs And Vaccines, Immunotherapeutics And Methods Of Using The Same | Not Available | 12-
Dec- 11 |
9-
May -18 |
22-
Nov -18 |
| US20180327
800 |
MONOCLONAL ANTIBODY PRODUCTION BY EBV TRANSFORMATION OF B CELLS | Not Available | 26-
Feb- 03 |
21-
May -18 |
15-
Nov -18 |
| US20180327
738 |
STABILIZED REAGENTS FOR GENOME MODIFICATION | Not Available | 20-
Nov- 15 |
18-
Nov -16 |
15-
Nov -18 |
| US20180327
697 |
CLEANING COMPOSITION, METHOD OF MAKING AND USE THEREOF | Not Available | 8-
Sep- 16 |
12-
Jun -18 |
15-
Nov -18 |
| US20180327
484 |
RSV-SPECIFIC BINDING MOLECULES AND MEANS FOR PRODUCING THEM | Not Available | 1-
Jun- 07 |
23-
Jul- 18 |
15-
Nov -18 |
| US20180326
070 |
CARBOHYDRATE CONJUGATES AS DELIVERY AGENTS FOR OLIGONUCLEOTIDES | Not Available | 4-
Dec- 07 |
20-
Nov -17 |
15-
Nov -18 |
| US20180326
051 |
LIPIDATED IMMUNE RESPONSE MODIFIER COMPOUND COMPOSITIONS, FORMULATIONS, AND METHODS | Not Available | 17-
Aug- 10 |
24-
Jul- 18 |
15-
Nov -18 |
| US20180326
045 |
COMBINATION PIV3/HMPV RNA VACCINES | ModernaTX, Inc. | 22-
Oct- 15 |
20-
Jul- 18 |
15-
Nov -18 |
| US20180326
044 |
NSP10 SELF-ASSEMBLING FUSION PROTEINS FOR VACCINES, THERAPEUTICS, DIAGNOSTICS AND OTHER NANOMATERIAL APPLICATIONS | Not Available | 13-
Oct- 15 |
13-
Oct -16 |
15-
Nov -18 |
| US20180326
039 |
VACCINE COMPOSITIONS | Not Available | 16-
Sep- 15 |
16-
Sep -16 |
15-
Nov -18 |
| US20180325
076 |
ANTIMICROBIAL COMPOSITIONS AND METHODS WITH NOVEL POLYMERIC BINDING SYSTEM | OXISCIENCE, LLC | 28-
Aug- 14 |
24-
Jul- 18 |
15-
Nov -18 |
| US20180321
242 |
VIRAL BIOMARKERS AND USES THEREFOR | Not Available | 6-
Nov- 15 |
4-
Nov -16 |
8-
Nov -18 |
| US20180319
811 |
DERIVATIVES OF PORPHYRINS, THEIR PROCESS OF PREPARATION AND THEIR USE FOR TREATING VIRAL INFECTIONS | Not Available | 30-
Oct- 15 |
28-
Oct -16 |
8-
Nov -18 |
| US20180319
779 |
SUBSTITUTED BENZOFURANYL AND BENZOXAZOLYL COMPOUNDS AND USES THEREOF | Not Available | 3-Jul-
13 |
4-
Dec -17 |
8-
Nov -18 |
| US20180318
447 |
COMPOSITIONS AND METHODS FOR IMPROVING VIRAL VECTOR EFFICIENCY | Not Available | 3-
Dec- 15 |
30-
Nov -16 |
8-
Nov -18 |
| US20180318
366 |
METHOD OF TREATMENT USING ONCOLYTIC VIRUSES | Not Available | 15-
Jun- 15 |
15-
Jun -16 |
8-
Nov -18 |
| US20180318
350 |
Immune Cells with DNMT3A Gene Modifications and Methods Related Thereto | Not Available | 4-
Nov- 15 |
4-
Nov -16 |
8-
Nov -18 |
| US20180312
575 |
ANTIBODIES AGAINST INFLUENZA VIRUS AND METHODS OF USE THEREOF | Not Available | 6-
Dec- 07 |
20-
Mar -18 |
1-
Nov -18 |
| US20180312
545 |
OPTIMIZED NUCLEIC ACID MOLECULES | Not Available | 9-
Nov- 15 |
9-
Nov -16 |
1-
Nov -18 |
| US20180312
544 |
RECOMBINANT HUMAN/BOVINE PARAINFLUENZA VIRUS 3 (B/HPIV3) EXPRESSING A CHIMERIC RSV/BPIV3 F PROTEIN AND USES THEREOF | The United States of America, as represented by the Secretary, Dept. of Health and Human Services | 20-
Jan- 15 |
20-
Jan -16 |
1-
Nov -18 |
| US20180311
338 |
MICRONEEDLE COMPOSITIONS AND METHODS OF USING SAME | Not Available | 11-
Jan- 16 |
11-
Jul- 18 |
1-
Nov -18 |
| US20180311
273 |
Method of Treating Inflammation | Not Available | 1-
Apr- 10 |
26-
Jun -18 |
1-
Nov -18 |
| US20180305
773 |
CRISPR EFFECTOR SYSTEM BASED DIAGNOSTICS FOR MALARIA DETECTION | Not Available | 12-
Apr- 17 |
12-
Apr -18 |
25-
Oct- 18 |
| US20180305
760 |
Pathogen biomarkers and uses therefor | Not Available | 30-
Sep- 15 |
30-
Sep -16 |
25-
Oct- 18 |
| US20180305
451 |
HIDE1 COMPOSITIONS AND METHODS | Not Available | 13-
Jul-15 |
13-
Jul- 16 |
25-
Oct- 18 |
| US20180305
412 |
COMPOSITIONS AND METHODS FOR TREATING DISEASES BY INHIBITING EXOSOME RELEASE | Not Available | 19-
Dec- 16 |
9-
Jul- 18 |
25-
Oct- 18 |
| US20180305
357 |
IMMUNE RESPONSE MODIFIER COMPOSITIONS AND METHDOS | Not Available | 22-
Dec- 06 |
25-
Jun -18 |
25-
Oct- 18 |
| US20180305
356 |
NOVEL KINASE INHIBITORS | Not Available | 19-
Oct- 12 |
18-
May -18 |
25-
Oct- 18 |
| US20180303
874 |
Compositions and Methods for the Prevention of Microbial Infections | Not Available | 10-
Nov- 11 |
30-
Nov -17 |
25-
Oct- 18 |
| US20180303
768 |
DESIGN, SYNTHESIS AND METHODS OF USE OF ACYCLIC FLEXMIER NUCLEOSIDE ANALOGUES HAVING ANTI-CORONAVIRUS ACTIVITY | Not Available | 30-
Jan- 15 |
2-
Jul- 18 |
25-
Oct- 18 |
| US20180303
090 |
TREATMENT COMPOSITIONS PROVIDING AN ANTIMICROBIAL BENEFIT | Not Available | 30-
Oct- 15 |
17-
Oct -16 |
25-
Oct- 18 |
| US20180267
031 |
METHOD AND DEVICE FOR DETECTING ANTIGEN-SPECIFIC ANTIBODIES IN A BIOLOGICAL FLUID SAMPLE BY USING NEODYMIUM MAGNETS | The U.S.A., as represented by the Secretary, Department of Health and Human Services | 1-
Sep- 15 |
8-
Aug -16 |
20-
Sep -18 |
| US20180265
847 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
29-
Mar -18 |
20-
Sep -18 |
| US20180265
822 |
LIQUID LOADING COMPOSITION, METHOD OF MAKING AND USE THEREOF | Not Available | 8-
Sep- 16 |
21-
May -18 |
20-
Sep -18 |
| US20180265
574 |
Anti-pneumococcal hyperimmune globulin for the treatment and prevention of pneumococcal infection | Not Available | 15-
Mar- 17 |
15-
Mar -17 |
20-
Sep -18 |
| US20180265
507 |
HOST TARGETED INHIBITORS OF DENGUE VIRUS AND OTHER VIRUSES | Dana-Farber Cancer Institute, Inc. | 11-
Apr- 12 |
26-
Jan -18 |
20-
Sep -18 |
| US20180264
098 |
MODULATION OF REPLICATIVE FITNESS BY DEOPTIMIZATION OF SYNONYMOUS CODONS | The Government of the USA as represented by the Secretary of the Dept. of Health and Human Service | 8-
Oct- 04 |
31-
May -18 |
20-
Sep -18 |
| US20180258
162 |
Methods Of Treating Inflammation Associated Airway Diseases And Viral Infections | Not Available | 2-
Jan- 15 |
22-
May -18 |
13-
Sep -18 |
| US20180258
160 |
Optimized Crosslinkers for Trapping a Target on a Substrate | Not Available | 13-
Nov- 15 |
11-
May -18 |
13-
Sep -18 |
| US20180258
159 |
COMPOSITIONS AND METHODS FOR THE TREATMENT OF IMMUNODEFICIENCY | Not Available | 28-
Oct- 14 |
14-
May -18 |
13-
Sep -18 |
| US20180258
151 |
RECOMBINANT SUPER-COMPOUND INTERFERON AND USES THEREOF | Not Available | 28-
Feb- 01 |
2-
Mar -18 |
13-
Sep -18 |
| US20180251
737 |
COMPOSITIONS, METHODS AND USES FOR INDUCING VIRAL GROWTH | Not Available | 5-
Dec- 08 |
28-
Dec -17 |
6-
Sep -18 |
| US20180251
540 |
HUMAN MONOCLONAL ANTIBODIES AGAINST INTERLEUKIN 8 (IL-8) | Not Available | 16-
Dec- 02 |
30-
Apr -18 |
6-
Sep -18 |
| US20180251
436 |
CERTAIN (2S)-N-[(1S)-1-CYANO-2-PHENYLETHYL]-1,4- OXAZEPANE-2-CARBOXAMIDES AS DIPEPTIDYL PEPTIDASE 1 INHIBITORS | Not Available | 24-
Jan- 14 |
19-
Sep -17 |
6-
Sep -18 |
| US20180250
602 |
PAPAYA MOSAIC VIRUS COMPOSITIONS AND USES THEREOF FOR STIMULATION OF THE INNATE IMMUNE RESPONSE | Not Available | 11-
Feb- 14 |
1-
May -18 |
6-
Sep -18 |
| US20180250
381 |
SOLUBLE NEEDLE ARRAYS FOR DELIVERY OF INFLUENZA VACCINES | Not Available | 20-
Aug- 10 |
12-
Oct -17 |
6-
Sep -18 |
| US20180245
056 |
COMPOSITIONS FOR INCREASING POLYPEPTIDE STABILITY AND ACTIVITY, AND RELATED METHODS | Not Available | 19-
Nov- 09 |
9-
Oct -17 |
30-
Aug -18 |
| US20180245
053 |
VIRAL VACCINES AND METHODS OF FORMING THE SAME | Not Available | 27-
Feb- 17 |
27-
Feb -18 |
30-
Aug -18 |
| US20180244
759 |
NOVEL METHODS OF GENERATING ANTIBODIES | Rutgers, The State University of New Jersey | 19-
Aug- 15 |
18-
Aug -16 |
30-
Aug -18 |
| US20180244
756 |
MIDDLE EAST RESPIRATORY SYNDROME CORONAVIRUS IMMUNOGENS, ANTIBODIES, AND THEIR USE | The United States of America, as Represented by the Secretary, Dept. of Health and Human Services | 24-
Feb- 15 |
24-
Feb -16 |
30-
Aug -18 |
| US20180244
669 |
IMIDAZO[4,5-c] RING COMPOUNDS CONTAINING SUBSTITUTED GUANIDINE GROUPS | Not Available | 31-
Aug- 15 |
26-
Aug -16 |
30-
Aug -18 |
| US20180244
660 |
CYCLOPROPYLDERIVATIVES AND THEIR USE AS KINASE INHIBITORS | Not Available | 17-
Aug- 15 |
17-
Aug -16 |
30-
Aug -18 |
| US20180243
347 |
IMMUNOMODULATORY COMPOSITIONS AND METHODS OF USE THEREOF | Not Available | 25-
Aug- 15 |
22-
Aug -16 |
30-
Aug -18 |
| US20180237
835 |
METHODS OF ANALYZING VIRUS-DERIVED THERAPEUTICS | American International Biotechnology, LLC | 31-
Jul-15 |
29-
Jul- 16 |
23-
Aug -18 |
| US20180237
788 |
IMPROVEMENTS IN OR RELATING TO DNA RECOMBINATION | The Regents of the University of California | 8-
Aug- 15 |
5-
Aug -16 |
23-
Aug -18 |
| US20180237
786 |
ARTIFICIAL NUCLEIC ACID MOLECULES | CUREVAC AG | 28-
Aug- 15 |
22-
Aug -16 |
23-
Aug -18 |
| US20180237
502 |
PAN-EBOLA AND PAN-FILOVIRUS PROTECTIVE EPITOPES, ANTIBODIES, AND ANTIBODY COCKTAILS | Integrated BioTherapeutics, Inc. | 11-
Mar- 15 |
11-
Mar -16 |
23-
Aug -18 |
| US20180237
435 |
GUANIDINE SUBSTITUTED IMIDAZO[4,5-c] RING COMPOUNDS | Not Available | 31-
Aug- 15 |
26-
Aug -16 |
23-
Aug -18 |
| US20180236
058 |
REVERSE GENETICS SYSTEMS | Not Available | 31-
Jul-09 |
16-
Oct -17 |
23-
Aug -18 |
| US20180236
054 |
Tetanus Toxoid and CCL3 Improve DC Vaccines | Duke University | 14-
Nov- 13 |
19-
Apr -18 |
23-
Aug -18 |
| US20180235
948 |
(S,E)-3-(6-AMINOPYRIDIN-3-YL)-N-((5-(4-(3-FLUORO-3- METHYLPYRROLIDINE-1-CARBONYL)PHENYL)-7-(4- FLUOROPHENYL)BENZOFURAN-2-YL)METHYL)ACRYLAMIDE FOR THE TREATMENT OF CANCER | Not Available | 18-
Aug- 15 |
18-
Aug -16 |
23-
Aug -18 |
| US20180230
521 |
BIOAGENT DETECTION OLIGONUCLEOTIDES | Not Available | 27-
Dec- 11 |
13-
Apr -18 |
16-
Aug -18 |
| US20180230
447 |
ACTIVE LOW MOLECULAR WEIGHT VARIANTS OF ANGIOTENSIN CONVERTING ENZYME 2 (ACE2) | Northwestern University | 24-
Jan- 17 |
24-
Jan -18 |
16-
Aug -18 |
| US20180228
695 |
DEVICES, SYSTEM AND METHOD TO CONTROL THE DELIVERY OF ORAL MEDICATIONS TO ENSURE THEY ARE EFFICACIOUS , TAKEN AS PRESCRIBED, AND TO AVOID UNWANTED SIDE EFFECTS | Not Available | 11-
Aug- 15 |
11-
Aug -16 |
16-
Aug -18 |
| US20180223
290 |
METHOD FOR PROPAGATING ADENOVIRAL VECTORS ENCODING INHIBITORY GENE PRODUCTS | GenVec, Inc. | 10-
Nov- 05 |
7-
Sep -17 |
9-
Aug -18 |
| US20180222
906 |
SUBSTITUTED IMIDAZOQUINOLINES, IMIDAZOPYRIDINES, AND IMIDAZONAPHTHYRIDINES | 3M Innovative Properties Company | 18-
Jun- 04 |
9-
Apr -18 |
9-
Aug -18 |
| US20180221
464 |
IMMUNOGENIC COMPOSITIONS, ANTIGEN SCREENING METHODS, AND METHODS OF GENERATING IMMUNE RESPONSES | Not Available | 3-
Aug- 15 |
3-
Aug -16 |
9-
Aug -18 |
| US20180216
164 |
HIGH DENSITY SELF-CONTAINED BIOLOGICAL ANALYSIS | Not Available | 15-
Nov- 06 |
19-
Mar -18 |
2-
Aug -18 |
| US20180216
067 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
29-
Mar -18 |
2-
Aug -18 |
| US20180215
831 |
Antibody Derivatives with Conditionally Enabled Effector Function | Not Available | 27-
Jul-15 |
27-
Jul- 16 |
2-
Aug -18 |
| US20180215
801 |
CRYPTIC POLYPEPTIDES AND USES THEREOF | Not Available | 29-
Jan- 15 |
29-
Jan -16 |
2-
Aug -18 |
| US20180215
794 |
TREATING CANCER WITH VIRAL NUCLEIC ACID | Mayo Foundation for Medical Education and Research | 20-
Feb- 07 |
27-
Mar -18 |
2-
Aug -18 |
| US20180214
430 |
Selective Inhibitors Of i-NOS For Use Against Viral Infection | UCL Business PLC | 17-
Jul-15 |
15-
Jul- 16 |
2-
Aug -18 |
| US20180209
960 |
Method of Determining, Identifying or Isolating Cell-Penetrating Peptides | Not Available | 23-
May- 11 |
11-
Dec -17 |
26-
Jul- 18 |
| US20180208
897 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
19-
Mar -18 |
26-
Jul- 18 |
| US20180208
659 |
ANTI-PD-L1 ANTIBODIES AND USES THEREOF | Not Available | 13-
Jun- 16 |
13-
Jun -17 |
26-
Jul- 18 |
| US20180208
653 |
METHODS FOR ENHANCING AN IMMUNE RESPONSE | Not Available | 20-
Jan- 17 |
19-
Jan -18 |
26-
Jul- 18 |
| US20180207
258 |
ADJUVANTED INFLUENZA VACCINES FOR PEDIATRIC USE | Not Available | 22-
Feb- 08 |
5-
Mar -18 |
26-
Jul- 18 |
| US20180207
145 |
PHARMACEUTICAL COMPOSITIONS COMPRISING DANIRIXIN FOR TREATING INFECTIOUS DISEASES | GlaxoSmithKline Intellectual Property (No. 2) Limited | 12-
May- 14 |
19-
Mar -18 |
26-
Jul- 18 |
| US20180201
998 |
COMPOSITIONS AND METHODS FOR DETECTION OF GENETIC DEAFNESS GENE MUTATION | CapitalBio Corporation | 14-
Jul-15 |
14-
Jul- 15 |
19-
Jul- 18 |
| US20180201
907 |
METHODS FOR INCREASING THE INFECTIVITY OF VIRUSES | Not Available | 26-
Jan- 12 |
13-
Mar -18 |
19-
Jul- 18 |
| US20180201
687 |
ANTIBODIES HAVING SPECIFICITY TO MYOSIN 18A AND USES THEREOF | Not Available | 7-Jul-
15 |
7-
Jul- 16 |
19-
Jul- 18 |
| US20180200
365 |
Methods and Compositions for Inhibiting Akt3 | Not Available | 17-
Jan- 17 |
20-
Feb -18 |
19-
Jul- 18 |
| US20180200
364 |
LIPIDATED IMMUNE RESPONSE MODIFIER COMPOUND COMPOSITIONS, FORMULATIONS, AND METHODS | 3M Innovative Properties Company | 17-
Aug- 10 |
27-
Nov -17 |
19-
Jul- 18 |
| US20180200
224 |
Antiviral Activity from Medicinal Mushrooms and Their Active Constituents | Not Available | 31-
Mar- 15 |
12-
Mar -18 |
19-
Jul- 18 |
| US20180200
196 |
Modular Particulars for Immunotherapy | Not Available | 1-
Nov- 13 |
3-
Jan -18 |
19-
Jul- 18 |
| US20180196
061 |
INFLUENZA POTENCY ASSAYS | Not Available | 7-Jul-
15 |
7-
Jul- 16 |
12-
Jul- 18 |
| US20180195
048 |
METHODS FOR PRODUCING VIRUS FOR VACCINE PRODUCTION | Takeda Vaccines, Inc. | 13-
Feb- 15 |
12-
Feb -16 |
12-
Jul- 18 |
| US20180194
850 |
ANTAGONISTIC ANTI-TUMOR NECROSIS FACTOR RECEPTOR SUPERFAMILY ANTIBODIES | Not Available | 15-
May- 15 |
13-
May -16 |
12-
Jul- 18 |
| US20180194
829 |
POLYPEPTIDES AND POLYNUCLEOTIDES, AND USES THEREOF FOR TREATMENT OF IMMUNE RELATED DISORDERS AND CANCER | Not Available | 15-
Apr- 11 |
13-
Nov -17 |
12-
Jul- 18 |
| US20180194
735 |
Sulfinylphenyl or Sulfonimidoylphenyl Benzazepines | Hoffmann La-Roche Inc. | 17-
Sep- 15 |
8-
Mar -18 |
12-
Jul- 18 |
| US20180193
477 |
DRUG-CONJUGATED BI-SPECIFIC ANTIGEN-BINDING CONSTRUCTS | Not Available | 15-
Jul-15 |
15-
Jul- 16 |
12-
Jul- 18 |
| US20180187
213 |
Variant AAV and Compositions, Methods and Uses for Gene Transfer to Cells, Organs and Tissues | The Children’s Hospital of Philadelphia | 22-
Jul-13 |
6-
Dec -17 |
5-
Jul- 18 |
| US20180187
211 |
METHODS AND COMPOSITIONS FOR COMBINATION IMMUNOTHERAPY | Not Available | 9-
Jan- 15 |
7-
Jan -16 |
5-
Jul- 18 |
| US20180187
165 |
HAND, FOOT, AND MOUTH VACCINES AND METHODS OF MANUFACTURE AND USE THEREOF | Takeda Vaccines, Inc. | 7-
Nov- 14 |
27-
Oct -17 |
5-
Jul- 18 |
| US20180187
154 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
20-
Feb -18 |
5-
Jul- 18 |
| US20180187
153 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
20-
Feb -18 |
5-
Jul- 18 |
| US20180187
131 |
DISINFECTING AQUEOUS FOAM, PROCESS FOR PREPARING SAME AND USE THEREOF | COMMISSARIAT A L’ENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES | 16-
Jun- 15 |
15-
Jun -16 |
5-
Jul- 18 |
| US20180186
897 |
NOVEL VACCINES IN PREVENTION AND TREATMENT OF MALARIA | Not Available | 26-
Jun- 15 |
24-
Jun -16 |
5-
Jul- 18 |
| US20180186
821 |
PROTEIN PROXIMITY ASSAY IN FORMALIN FIXED PAFFAFIN EMBEDDED TISSUE USING CAGED HAPTENS | Not Available | 28-
Aug- 15 |
28-
Feb -18 |
5-
Jul- 18 |
| US20180186
802 |
COMPOUNDS AND COMPOSITIONS AS TOLL-LIKE RECEPTOR 7 AGONISTS | Not Available | 1-
May- 14 |
27-
Feb -18 |
5-
Jul- 18 |
| US20180186
792 |
HETEROBIFUNCTIONAL LINKERS WITH POLYETHYLENE GLYCOL SEGMENTS AND IMMUNE RESPONSE MODIFIER CONJUGATES MADE THEREFROM | 3M Innovative Properties Company | 3-
Jun- 11 |
26-
Feb -18 |
5-
Jul- 18 |
| US20180186
534 |
Powdered Pouch And Method Of Making Same | MONOSOL, LLC | 16-
Apr- 12 |
29-
Dec -17 |
5-
Jul- 18 |
| US20180185
469 |
COMPOSITIONS AND METHODS FOR TREATING AND PREVENTING PORCINE REPRODUCTIVE AND RESPIRATORY SYNDROME | Not Available | 24-
Apr- 12 |
14-
Dec -17 |
5-
Jul- 18 |
| US20180185
392 |
Pharmaceutical Compositions and Methods | Not Available | 3-
Aug- 15 |
29-
Nov -17 |
5-
Jul- 18 |
| US20180185
345 |
METHODS AND COMPOSITIONS FOR TREATING HERPESVIRUS INDUCED CONDITIONS | Not Available | 19-
Jun- 15 |
17-
Jun -16 |
5-
Jul- 18 |
| US20180180
544 |
USE OF A FLUORESCENT MATERIAL TO DETECT FAILURE OR DETERIORATED PERFORMANCE OF A FLUOROMETER | Not Available | 14-
Jun- 12 |
22-
Feb -18 |
28-
Jun- 18 |
| US20180179
300 |
GENERATION OF BINDING MOLECULES | Merus N.V. | 26-
Sep- 11 |
22-
Nov -17 |
28-
Jun- 18 |
| US20180179
274 |
PROTEINS COMPRISING A MUTATED LAIR-1 FRAGMENT AND USES THEREOF | Not Available | 26-
Jun- 15 |
24-
Jun -16 |
28-
Jun- 18 |
| US20180177
863 |
METHODS OF MAKING AND USING LIVE ATTENUATED VIRUSES | Not Available | 23-
Sep- 15 |
31-
Oct -17 |
28-
Jun- 18 |
| US20180177
862 |
ANTIGENICALLY MATCHED INFLUENZA VACCINES | Not Available | 26-
Jun- 15 |
24-
Jun -16 |
28-
Jun- 18 |
| US20180177
860 |
VACCINE CONTAINING VIRUS INACTIVATED BY GREEN TEA EXTRACT, AND PREPARATION METHOD THEREFOR | Not Available | 11-
Jun- 15 |
7-
Jun -16 |
28-
Jun- 18 |
| US20180163
182 |
PROCESSES FOR PRODUCTION AND PURIFICATION OF NUCLEIC ACID-CONTAINING COMPOSITIONS | Human Services | 10-
Jun- 15 |
10-
Jun -16 |
14-
Jun- 18 |
| US20180162
838 |
Chemical Compounds | Not Available | 18-
Mar- 14 |
13-
Dec -17 |
14-
Jun- 18 |
| US20180162
835 |
QUINAZOLINONES AND AZAQUINAZOLINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
11-
Dec -17 |
14-
Jun- 18 |
| US20180161
425 |
NOVEL PROTEIN STRUCTURE USED FOR EFFICIENT ANTIBODY PRODUCTION IN IMMUNIZATION | Not Available | 10-
Dec- 14 |
10-
Dec -15 |
14-
Jun- 18 |
| US20180161
422 |
NUCLEIC ACID COMPRISING OR CODING FOR A HISTONE STEM- LOOP AND A POLY(A) SEQUENCE OR A POLYADENYLATION SIGNAL FOR INCREASING THE EXPRESSION OF AN ENCODED PATHOGENIC ANTIGEN | CureVac AG | 15-
Feb- 12 |
8-
Feb -18 |
14-
Jun- 18 |
| US20180161
279 |
GASTRO-RETENTIVE MODIFIED RELEASE DOSAGE FORMS FOR OPROZOMIB AND PROCESS TO MAKE THEREOF | Not Available | 14-
Dec- 16 |
13-
Dec -17 |
14-
Jun- 18 |
| US20180160
662 |
Transgenic Immunodeficient Mouse Expressing Human SIRP-alpha | Institut Pasteur | 26-
Mar- 12 |
25-
Jan -18 |
14-
Jun- 18 |
| US20180149
659 |
DIAGNOSIS AND TREATMENT OF MERS-RELATED RENAL DISEASE | Not Available | 4-
Jun- 15 |
3-
Jun -16 |
31-
May -18 |
| US20180148
727 |
ARTIFICIAL NUCLEIC ACID MOLECULES | Not Available | 30-
Dec- 14 |
29-
Dec -15 |
31-
May -18 |
| US20180142
239 |
METHODS AND COMPOSITIONS FOR THE TREATMENT OF CANCER OR OTHER DISEASES | Not Available | 26-
Jan- 07 |
14-
Jun -17 |
24-
May -18 |
| US20180142
198 |
DELIVERY OF BIOMOLECULES TO IMMUNE CELLS | Not Available | 31-
Oct- 14 |
30-
Oct -15 |
24-
May -18 |
| US20180142
006 |
ANTIBODY PRODUCING NON-HUMAN ANIMALS | Merus N.V. | 27-
Jun- 08 |
12-
Jan -18 |
24-
May -18 |
| US20180142
005 |
ANTIBODY PRODUCING NON-HUMAN ANIMALS | Merus N.V. | 27-
Jun- 08 |
12-
Jan -18 |
24-
May -18 |
| US20180142
004 |
ANTIBODY PRODUCING NON-HUMAN ANIMALS | Merus N.V. | 27-
Jun- 08 |
11-
Jan -18 |
24-
May -18 |
| US20180142
003 |
ANTIBODY PRODUCING NON-HUMAN ANIMALS | Merus N.V. | 27-
Jun- 08 |
9-
Jan -18 |
24-
May -18 |
| US20180142
002 |
ANTIBODY PRODUCING NON-HUMAN ANIMALS | Merus N.V. | 27-
Jun- 08 |
5-
Jan -18 |
24-
May -18 |
| US20180140
659 |
ANALOGS OF C5a AND METHODS OF USING SAME | Not Available | 29-
Jun- 10 |
12-
Jan -18 |
24-
May -18 |
| US20180140
625 |
PRODUCTION OF STABLE NON-POLYADENYLATED RNAS | Massachusetts Institute of Technology | 16-
Oct- 12 |
31-
Jul- 17 |
24-
May -18 |
| US20180140
580 |
METHOD OF TREATING OR INHIBITING THE DEVELOPMENT OF BRAIN INFLAMMATION AND SEPSIS | Not Available | 29-
Nov- 02 |
23-
Oct -17 |
24-
May -18 |
| US20180135
099 |
NANOREPORTERS AND METHODS OF MANUFACTURING AND USE THEREOF | Not Available | 23-
Dec- 05 |
2-
Jan -18 |
17-
May -18 |
| US20180135
012 |
MEMBRANE-RECEIVER COMPLEX THERAPEUTICS | Not Available | 13-
May- 15 |
13-
May -16 |
17-
May -18 |
| US20180134
783 |
HUMAN MONOCLONAL ANTIBODIES AGAINST INTERLEUKIN 8 (IL-8) | Not Available | 16-
Dec- 02 |
26-
Apr -16 |
17-
May -18 |
| US20180134
770 |
ANTIBODY PRODUCING NON-HUMAN ANIMALS | Merus N.V. | 27-
Jun- 08 |
12-
Jan -18 |
17-
May -18 |
| US20180133
246 |
INHALATION OF NITRIC OXIDE FOR TREATING RESPIRATORY DISEASES | Not Available | 7-
Mar- 12 |
1-
Nov -17 |
17-
May -18 |
| US20180127
836 |
IMPROVED COMPOSITIONS AND METHODS FOR DETECTION OF VIRUSES | Not Available | 7-
May- 15 |
6-
May -16 |
10-
May -18 |
| US20180127
783 |
CRISPR-RELATED METHODS AND COMPOSITIONS WITH GOVERNING gRNAS | EDITAS MEDICINE, INC. | 7-
Nov- 13 |
29-
Nov -17 |
10-
May -18 |
| US20180127
384 |
HYDRAZIDE CONTAINING NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 29-
Jul-11 |
21-
Jun -17 |
10-
May -18 |
| US20180125
965 |
HAND, FOOT, AND MOUTH VACCINES AND METHODS OF MANUFACTURE AND USE THEREOF | Takeda Vaccines, Inc. | 7-
Nov- 14 |
6-
Nov -15 |
10-
May -18 |
| US20180125
952 |
PRIME-BOOST REGIMENS INVOLVING ADMINISTRATION OF AT LEAST ONE mRNA CONSTRUCT | Not Available | 15-
May- 15 |
13-
May -16 |
10-
May -18 |
| US20180125
883 |
INHALATION OF NITRIC OXIDE FOR TREATING RESPIRATORY DISEASES | Not Available | 7-
Mar- 12 |
20-
Sep -17 |
10-
May -18 |
| US20180112
270 |
C-CBL MUTATIONS AND USES THEREOF | Not Available | 4-
Jun- 10 |
22-
Mar -16 |
26-
Apr- 18 |
| US20180111
991 |
MODULATORS OF ACTIVIN AND METHODS FOR MODULATING IMMUNE RESPONSES AND T FOLLICULAR HELPER CELLS | Not Available | 2-
Dec- 14 |
2-
Jun -17 |
26-
Apr- 18 |
| US20180U1
907 |
DENDRIMER LIKE AMINO AMIDES POSSESSING SODIUM CHANNEL BLOCKER ACTIVITY FOR THE TREATMENT OF DRY EYE AND OTHER
MUCOSAL DISEASES |
Not Available | 29-
May- 12 |
20-
Dec -17 |
26-
Apr- 18 |
| US20180110
845 |
METHOD OF PROVIDING PATIENT SPECIFIC IMMUNE RESPONSE IN AMYLOIDOSES AND PROTEIN AGGREGATION DISORDERS | Not Available | 31-
Aug- 07 |
18-
Dec -17 |
26-
Apr- 18 |
| US20180105
815 |
Bivalent siRNA Chimeras and Methods of Use Thereof | Not Available | 18-
Oct- 16 |
6-
Oct -17 |
19-
Apr- 18 |
| US20180105
596 |
ANTI-TYRO3 ANTIBODIES AND USES THEREOF | Not Available | 17-
Apr- 15 |
15-
Apr -16 |
19-
Apr- 18 |
| US20180105
514 |
HETEROCYCLIC AMIDES USEFUL AS PROTEIN MODULATORS | Not Available | 7-
Apr- 16 |
3-
Jan -18 |
19-
Apr- 18 |
| US20180104
241 |
CHEMICALLY AND METABOLICALLY STABLE DIPEPTIDE POSSESSING POTENT SODIUM CHANNEL BLOCKER ACTIVITY | PARION SCIENCES, INC. | 27-
Jun- 11 |
15-
Dec -17 |
19-
Apr- 18 |
| US20180100
181 |
METHODS FOR DETECTING AGGLUTINATION AND COMPOSITIONS FOR USE IN PRACTICING THE SAME | Not Available | 17-
Apr- 15 |
15-
Apr -16 |
12-
Apr- 18 |
| US20180099
999 |
FUSION PROTEINS, RECOMBINANT BACTERIA, AND METHODS FOR USING RECOMBINANT BACTERIA | Not Available | 17-
Sep- 14 |
14-
Dec -17 |
12-
Apr- 18 |
| US20180098
972 |
TREATMENT OF INFECTIOUS DISEASES | CHILDREN’S MEDICAL CENTER CORPORATION | 26-
Jan- 15 |
26-
Jan -16 |
12-
Apr- 18 |
| US20180092
932 |
Anti-Viral Azide Containing Compounds | Not Available | 28-
Jul-10 |
7-
Dec -17 |
5-
Apr- 18 |
| US20180087
049 |
MAXIMIZING DNA YIELD OF BLOOD SPECIMENS COLLECTED IN RAPID CLOT TUBES | Not Available | 27-
Sep- 16 |
7-
Sep -17 |
29-
Mar -18 |
| US20180086
818 |
COMPOSITIONS AND METHODS FOR THE TREATMENT OF IMMUNODEFICIENCY | Not Available | 28-
Oct- 14 |
13-
Nov -17 |
29-
Mar -18 |
| US20180085
457 |
ANTIBODY/T-CELL RECEPTOR CHIMERIC CONSTRUCTS AND USES THEREOF | Not Available | 23-
Oct- 15 |
1-
Dec -17 |
29-
Mar -18 |
| US20180085
432 |
STING (Stimulator of Interferon Genes), A Regulator of Innate Immune Responses | Not Available | 4-
Aug- 08 |
15-
Sep -17 |
29-
Mar -18 |
| US20180085
388 |
DELIVERY OF RNA TO TRIGGER MULTIPLE IMMUNE PATHWAYS | GLAXOSMITHKLINE BIOLOGICALS, SA | 6-Jul-
10 |
5-
Oct -17 |
29-
Mar -18 |
| US20180079
746 |
HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS | Not Available | 19-
Mar- 15 |
15-
Mar -16 |
22-
Mar -18 |
| US20180078
625 |
COMPOSITIONS AND METHODS FOR DELIVERY OF BIOMACROMOLECULE AGENTS | Not Available | 25-
Mar- 15 |
25-
Mar -16 |
22-
Mar -18 |
| US20180078
532 |
IMMEDIATE RELEASE FORMULATIONS FOR OPROZOMIB | AMGEN INC. | 21-
Sep- 16 |
18-
Sep -17 |
22-
Mar -18 |
| US20180078
507 |
BIODEGRADABLE POLYMERIC PARTICLES ENCAPSULATING AN ACTIVE AGENT, PHARMACEUTICAL COMPOSITIONS AND USES THEREOF | Not Available | 16-
Sep- 16 |
15-
Sep -17 |
22-
Mar -18 |
| US20180073
073 |
METHODS AND COMPOSITIONS FOR LABELING TARGETS AND HAPLOTYPE PHASING | Not Available | 18-
Mar- 15 |
16-
Mar -16 |
15-
Mar -18 |
| US20180072
813 |
CARBONIC ANHYDRASE IX (G250) ANTIBODIES AND METHODS OF USE THEREOF | Not Available | 2-
Dec- 05 |
9-
May -17 |
15-
Mar -18 |
| US20180072
796 |
MAST CELL STABILIZERS FOR TREATMENT OF HYPERCYTOKINEMIA AND VIRAL INFECTION | Not Available | 8-
Sep- 16 |
17-
Nov -17 |
15-
Mar -18 |
| US20180072
752 |
COUMARIN DERIVATIVE AS ANTIVIRAL AGENT, PHARMACEUTICAL COMPOSITION THEREOF, ITS PREPARATION AND USE | Not Available | 30-
Mar- 15 |
2-
Feb -16 |
15-
Mar -18 |
| US20180071
219 |
Technology for Preparation of Macromolecular Microspheres | Not Available | 24-
Jan- 06 |
25-
Sep -17 |
15-
Mar -18 |
| US20180067
299 |
ENDOSCOPIC APPARATUS FOR THERMAL DISTRIBUTION MONITORING | ELECTRONICS AND TELECOMMUNICATIONS RESEARCH INSTITUTE | 7-
Sep- 16 |
31-
May -17 |
8-
Mar -18 |
| US20180066
228 |
Detection of T Cell Exhaustion or Lack of T Cell Costimulation and Uses Thereof | Not Available | 15-
May- 15 |
15-
Nov -17 |
8-
Mar -18 |
| US20180066
216 |
CLEANING COMPOSITION, METHOD OF MAKING AND USE THEREOF | Not Available | 8-
Sep- 16 |
6-
Sep -17 |
8-
Mar -18 |
| US20180065
981 |
HETEROCYCLYLMETHYL-THIENOURACILE AS ANTAGONISTS OF THE ADENOSINE-A2B-RECEPTOR | Not Available | 26-
Mar- 15 |
21-
Mar -16 |
8-
Mar -18 |
| US20180064
790 |
Composition for Treatment or Prevention of Infectious Inflammatory Diseases, or Composition for Immune Enhancement, Comprising Tryptophanyl-tRNA Synthetase as an Active Ingredient | Not Available | 26-
Feb- 15 |
25-
Aug -17 |
8-
Mar -18 |
| US20180064
752 |
ANIONICALLY MODIFIED POLYALLYLAMINE DERIVATIVE, USE OF ANIONICALLY MODIFIED POLYALLYLAMINE DERIVATIVE AS MEDICINE, PARTICULARLY FOR PROPYLAXIS AND TREATMENT OF INFECTIONS OF RESPIRATORY TRACT CAUSED BY HUMAN METAPNEUMOVIRUS (HMPV), HUMAN RHINOVIRUSES (HRV), AND INFECTION BY INFLUENZA VIRUS TYPE A (IAV) AND PHARMACEUTICAL COMPOSITION COMPRISING THE ANIONICALLY MODIFIED POLYALLYLAMINE DERIVATIVE | Not Available | 29-
Jul-14 |
25-
Oct -17 |
8-
Mar -18 |
| US20180058
988 |
SAMPLE FIXATION AND STABILISATION | Not Available | 1-
Mar- 13 |
14-
Aug -17 |
1-
Mar -18 |
| US20180057
871 |
COMPOSITIONS AND METHODS FOR DETECTING RARE SEQUENCE VARIANTS | Not Available | 15-
Aug- 16 |
1-
Nov -17 |
1-
Mar -18 |
| US20180057
841 |
METHOD OF INCREASING THE FUNCTION OF AN AAV VECTOR | Not Available | 7-
Apr- 05 |
27-
Oct -17 |
1-
Mar -18 |
| US20180057
817 |
Particle-Nucleic Acid Conjugates and Therapeutic Uses Related Thereto | Not Available | 25-
Jun- 12 |
9-
Oct -17 |
1-
Mar -18 |
| US20180057
594 |
PSEUDOTYPED ONCOLYTIC VIRAL DELIVERY OF THERAPEUTIC POLYPEPTIDES | Not Available | 30-
Jun- 16 |
29-
Sep -17 |
1-
Mar -18 |
| US20180057
509 |
ALKYLOXY SUBSTITUTED THIAZOLOQUINOLINES AND THIAZOLONAPHTHYRIDINES | Not Available | 9-
Feb- 05 |
2-
Nov -17 |
1-
Mar -18 |
| US20180057
488 |
COMPOSITIONS AND METHODS FOR INHIBITING KINASES | Not Available | 23-
Apr- 15 |
7-
Nov -17 |
1-
Mar -18 |
| US20180055
925 |
DISPLAY PLATFORM FROM BACTERIAL SPORE COAT PROTEINS | The United States of America, as represented by the Secretary, Department of Health and Human Serv | 3-
Mar- 15 |
7-
Aug -15 |
1-
Mar -18 |
| US20180055
769 |
CIRCULATION OF COMPONENTS DURING MICROFLUIDIZATION AND/OR HOMOGENIZATION OF EMULSIONS | Not Available | 3-
Dec- 09 |
26-
Jun -17 |
1-
Mar -18 |
| US20180051
267 |
TAL EFFECTOR-MEDIATED DNA MODIFICATION | Not Available | 10-
Dec- 09 |
5-
Oct -17 |
22-
Feb -18 |
| US20180051
266 |
TAL EFFECTOR-MEDIATED DNA MODIFICATION | Not Available | 10-
Dec- 09 |
21-
Aug -17 |
22-
Feb -18 |
| US20180050
059 |
DELIVERY OF RNA TO DIFFERENT CELL TYPES | GLAXOSMITHKLINE BIOLOGICALS, SA | 6-Jul-
10 |
30-
Aug -17 |
22-
Feb -18 |
| US20180044
687 |
ARTIFICIAL NUCLEIC ACID MOLECULES FOR IMPROVED PROTEIN EXPRESSION | Not Available | 12-
Dec- 14 |
11-
Dec -15 |
15-
Feb -18 |
| US20180044
328 |
PEPTIDYL NITRIL COMPOUNDS AS DIPEPTIDYL PEPTIDASE I INHIBITORS | Prozymex A/S | 5-
Mar- 15 |
4-
Mar -16 |
15-
Feb -18 |
| US20180043
007 |
INFLUENZA VIRUS VECTORS AND USES THEREFOR | Not Available | 17-
Mar- 14 |
21-
Aug -17 |
15-
Feb -18 |
| US20180037
952 |
SYSTEM AND METHOD FOR DNA SEQUENCING AND BLOOD CHEMISTRY ANALYSIS | Nanomedical Diagnostics, Inc. | 28-
Apr- 14 |
21-
Aug -17 |
8-
Feb -18 |
| US20180037
942 |
ENZYME-INDEPENDENT MOLECULAR INDEXING | Not Available | 3-
Aug- 16 |
1-
Aug -17 |
8-
Feb -18 |
| US20180037
871 |
CANCER INITIATING CELL AND USE THEREOF | Not Available | 8-
Aug- 16 |
8-
Aug -16 |
8-
Feb -18 |
| US20180037
636 |
STRUCTURED VIRAL PEPTIDE COMPOSITIONS AND METHODS OF USE | DANA-FARBER CANCER INSTITUTE, INC. | 18-
Jun- 09 |
21-
Sep -17 |
8-
Feb -18 |
| US20180037
634 |
ENGINEERED POLYPEPTIDES AND USES THEREOF | Not Available | 2-
Aug- 16 |
2-
Aug -17 |
8-
Feb -18 |
| US20180037
617 |
METHODS AND COMPOSITIONS FOR TREATING AND/OR PREVENTING A DISEASE OR DISORDER ASSOCIATED WITH ABNORMAL LEVEL AND/OR ACTIVITY OF THE IFP35 FAMILY OF PROTEINS | Institute of Biophysics, Chinese Academy of Sciences | 22-
Aug- 14 |
21-
Aug -15 |
8-
Feb -18 |
| US20180036
398 |
FLAVIVIRUS REPLICONS | Not Available | 27-
Feb- 15 |
25-
Feb -16 |
8-
Feb -18 |
| US20180036
237 |
OIL/SURFACTANT MIXTURES FOR SELF-EMULSIFICATION | GLAXOSMITHKLINE BIOLOGICALS, SA | 23-
Feb- 15 |
23-
Feb -16 |
8-
Feb -18 |
| US20180031
555 |
METHOD FOR SELECTING A SINGLE CELL EXPRESSING A HETEROGENEOUS COMBINATION OF ANTIBODIES | Menus N.V. | 27-
Jun- 08 |
18-
Jul- 17 |
1-
Feb -18 |
| US20180030
429 |
Polypeptide Assemblies and Methods for the Production Thereof | Not Available | 27-
Feb- 15 |
29-
Feb -16 |
1-
Feb -18 |
| US20180030
411 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
13-
Oct -17 |
1-
Feb -18 |
| US20180028
677 |
Peptides for Assisting Delivery Across the Blood Brain Barrier | Children’s Medical Center Corporation | 22-
May- 06 |
30-
Jun -17 |
1-
Feb -18 |
| US20180028
626 |
IMMUNOTHERAPEUTIC VACCINE AND ANTIBODY COMBINATION THERAPY | Tnansgene SA | 13-
Feb- 15 |
12-
Feb -16 |
1-
Feb -18 |
| US20180028
562 |
METHODS OF TREATING OR PREVENTING INFLAMMATION AND HYPERSENSITIVITY WITH OXIDATIVE REDUCTIVE POTENTIAL
WATER SOLUTION |
SONOMA PHARMACEUTICALS, INC. | 20-
Jan- 06 |
10-
Oct -17 |
1-
Feb -18 |
| US20180028
449 |
Technology for the Preparation of Microparticles | Not Available | 24-
Jul-07 |
27-
Jun -17 |
1-
Feb -18 |
| US20180028
431 |
POLYMER-BASED ANTIMICROBIAL COMPOSITIONS AND METHODS OF USE THEREOF | eXion labs Inc. | 28-
Jul-16 |
27-
Jul- 17 |
1-
Feb -18 |
| US20180023
048 |
ANIMAL PROTEIN-FREE MEDIA FOR CULTIVATION OF CELLS | Not Available | 29-
Oct- 04 |
22-
Sep -17 |
25-
Jan- 18 |
| US20180022
781 |
POLYPEPTIDES FOR ENGINEERING INTEGRASE CHIMERIC PROTEINS AND THEIR USE IN GENE THERAPY | CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (CNRS) | 13-
Feb- 15 |
12-
Feb -16 |
25-
Jan- 18 |
| US20180021
448 |
Conjugates of Cell Binding Molecules with Cytotoxic Agents | Hangzhou DAC Biotech Co., Ltd. | 12-
Jul-12 |
16-
Apr -14 |
25-
Jan- 18 |
| US20180016
307 |
GRIFFITHSIN MUTANTS | The United States of America, as represented by the Secretary, Department of Health and Human Serv | 10-
Feb- 15 |
10-
Feb -16 |
18-
Jan- 18 |
| US20180016
285 |
Boron-Containing Small Molecules | Not Available | 20-
Jun- 07 |
28-
Sep -17 |
18-
Jan- 18 |
| US20180016
243 |
HUMAN HELICASE DDX3 INHIBITORS AS THERAPEUTIC AGENTS | Not Available | 13-
Feb- 15 |
12-
Feb -16 |
18-
Jan- 18 |
| US20180015
174 |
SYNTHETIC NANOPARTICLES FOR DELIVERY OF IMMUNOMODULATORY COMPOUNDS | Not Available | 15-
Jul-16 |
14-
Jul- 17 |
18-
Jan- 18 |
| US20180015
052 |
DESIGN, SYNTHESIS AND METHODS OF USE OF ACYCLIC FLEXMIER NUCLEOSIDE ANALOGUES HAVING ANTI-CORONAVIRUS ACTIVITY | Not Available | 30-
Jan- 15 |
28-
Jan -16 |
18-
Jan- 18 |
| US20180010
167 |
ORGANISM IDENTIFICATION PANEL | Not Available | 2-
Apr- 07 |
26-
Jul- 17 |
11-
Jan- 18 |
| US20180010
125 |
DOUBLE-STRANDED OLIGONUCLEOTIDE MOLECULES TO DDIT4 AND METHODS OF USE THEREOF | Quark Pharmaceuticals Inc. | 12-
Sep- 12 |
15-
Feb -17 |
11-
Jan- 18 |
| US20180009
787 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.P.A. | 31-
May- 16 |
22-
Sep -17 |
11-
Jan- 18 |
| US20180008
689 |
RNA VIRUS ATTENUATION BY ALTERATION OF MUTATIONAL ROBUSTNESS AND SEQUENCE SPACE | Not Available | 28-
Jan- 15 |
28-
Jan -16 |
11-
Jan- 18 |
| US20180002
743 |
FLUOROGENIC PROBES AND THEIR USE IN QUANTITATIVE DETECTION OF TARGET RNA SEQUENCES | Not Available | 18-
Jun- 16 |
16-
Jun -17 |
4-
Jan- 18 |
| US20180002
406 |
NEUTRALIZING GP41 ANTIBODIES AND THEIR USE | The United States of America, as represented by the Secretary, Department of Health and Human | 7-
Nov- 11 |
8-
Sep -17 |
4-
Jan- 18 |
| US20180000
929 |
D-AMINO ACID DERIVATIVE-MODIFIED PEPTIDOGLYCAN AND METHODS OF USE THEREOF | Not Available | 30-
Nov- 12 |
14-
Sep -17 |
4-
Jan- 18 |
| US20180000
926 |
METHODS OF INDUCING AN IMMUNE RESPONSE TO HEPATITIS C VIRUS | Not Available | 15-
Jan- 15 |
15-
Jan -16 |
4-
Jan- 18 |
| US20180000
868 |
Induced Hepatocytes and Uses Thereof | Not Available | 26-
Nov- 14 |
15-
Sep -17 |
4-
Jan- 18 |
| US20180000
724 |
METHODS FOR INDUCING AN IMMUNE RESPONSE VIA BUCCAL AND/OR SUBLINGUAL ADMINISTRATION OF A VACCINE | Not Available | 26-
Jul-10 |
10-
May -17 |
4-
Jan- 18 |
| US20170369
843 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
29-
Mar -17 |
28-
Dec -17 |
| US20170369
470 |
Cyclic Compounds and Uses Thereof | Not Available | 16-
Dec- 14 |
16-
Dec -15 |
28-
Dec -17 |
| US20170368
203 |
Compositions For Enhancing Transport Of Molecules Into Cells | Not Available | 29-
Apr- 03 |
10-
Jan -17 |
28-
Dec -17 |
| US20170368
201 |
SCALABLE MANUFACTURING PROCESS TO PRODUCE RECOMBINANT LENTIVIRAL VECTORS IN SERUM-FREE SUSPENSION CELL CULTURE SYSTEM | The Children’s Hospital of Philadelphia | 15-
Mar- 13 |
8-
Sep -17 |
28-
Dec -17 |
| US20170368
167 |
COMPOSITIONS AND METHODS RELATED TO NEUROLOGICAL DISORDERS | Not Available | 21-
Aug- 12 |
6-
Jun -16 |
28-
Dec -17 |
| US20170362
300 |
OLIGOPEPTIDE-FREE CELL CULTURE MEDIA | Not Available | 4-
Jan- 06 |
7-
Aug -17 |
21-
Dec -17 |
| US20170362
297 |
CHIMERIC ANTIGEN RECEPTORS AND METHODS OF USE THEREOF | Not Available | 19-
Dec- 14 |
21-
Dec -15 |
21-
Dec -17 |
| US20170362
187 |
3,5-DIAMINO-6-CHLORO-N-(N-(4-PHENYLBUTYL)CARBAMIMIDOYL) PYRAZINE-2- CARBOXAMIDE COMPOUNDS | Parion Sciences, Inc. | 17-
Dec- 12 |
29-
Jun -17 |
21-
Dec -17 |
| US20170362
170 |
HEPATITIS C ANTIVIRAL COMPOSITIONS AND METHODS | Not Available | 3-
Aug- 07 |
29-
Jun -17 |
21-
Dec -17 |
| US20170360
962 |
NOVEL RECOMBINANT ADENO-ASSOCIATED VIRUS CAPSIDS RESISTANT TO PRE-EXISTING HUMAN NEUTRALIZING ANTIBODIES | Not Available | 16-
Feb- 16 |
18-
Aug -17 |
21-
Dec -17 |
| US20170360
960 |
AAV Vectors Targeted to the Central Nervous System | Not Available | 21-
Nov- 14 |
20-
Nov -15 |
21-
Dec -17 |
| US20170360
908 |
VACCINE PHARMACEUTICAL COMPOSITION FOR CELL-MEDIATED IMMUNITY CONTAINING BISPHOSPHONATES | NITTO DENKO CORPORATION | 3-
Sep- 14 |
2-
Sep -15 |
21-
Dec -17 |
| US20170360
881 |
PEPTIDOMIMETIC MACROCYCLES AND USES THEREOF | Not Available | 17-
Jun- 16 |
16-
Jun -17 |
21-
Dec -17 |
| US20170360
875 |
METHODS FOR TREATING IMMUNE-MEDIATED VIRAL INFECTIONS | MIDDLE TENNESSEE STATE UNIVERSITY | 22-
Dec- 14 |
22-
Dec -15 |
21-
Dec -17 |
| US20170358
082 |
STAIN-FREE HISTOPATHOLOGY BY CHEMICAL IMAGING | Not Available | 15-
Mar- 13 |
2-
Aug -17 |
14-
Dec -17 |
| US20170354
727 |
MODULATION OF REPLICATIVE FITNESS BY DEOPTIMIZATION OF SYNONYMOUS CODONS | Not Available | 8-
Oct- 04 |
23-
Aug -17 |
14-
Dec -17 |
| US20170348
433 |
NOVEL RECOMBINANT ADENO-ASSOCIATED VIRUS CAPSIDS RESISTANT TO PRE-EXISTING HUMAN NEUTRALIZING ANTIBODIES | The Board of Trustees of the Leland Stanford Junior University | 16-
Feb- 16 |
16-
Feb -17 |
7-
Dec -17 |
| US20170348
402 |
SYSTEM AND METHOD FOR DELIVERING GENETIC MATERIAL OR PROTEIN TO CELLS | Not Available | 30-
Jul-14 |
30-
Jul- 15 |
7-
Dec -17 |
| US20170348
369 |
Plant Extract and Its Therapeutic Use | Not Available | 16-
May- 08 |
18-
Jul- 17 |
7-
Dec -17 |
| US20170342
442 |
RECOMBINANT SELF-REPLICATING POLYCISTRONIC RNA MOLECULES | GLAXOSMITHKLINE BIOLOGICALS, SA | 11-
Oct- 11 |
15-
Aug -17 |
30-
Nov -17 |
| US20170342
405 |
MOLECULAR INDEXING OF INTERNAL SEQUENCES | Not Available | 31-
May- 16 |
16-
May -17 |
30-
Nov -17 |
| US20170342
056 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.P.A. | 31-
May- 16 |
26-
May -17 |
30-
Nov -17 |
| US20170340
735 |
Anti-TIGIT Antigen-Binding Proteins and Methods of Use Thereof | Not Available | 1-
Oct- 15 |
19-
Jun -17 |
30-
Nov -17 |
| US20170340
725 |
COMBINATION PIV3/HMPV RNA VACCINES | ModernaTX, Inc. | 22-
Oct- 15 |
11-
Aug -17 |
30-
Nov -17 |
| US20170340
721 |
METHODS AND COMPOSITIONS FOR ENHANCING IMMUNE RESPONSES | Not Available | 3-
Sep- 14 |
3-
Sep -15 |
30-
Nov -17 |
| US20170340
611 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.P.A. | 31-
May- 16 |
26-
May -17 |
30-
Nov -17 |
| US20170337
459 |
SPATIALLY ADDRESSABLE MOLECULAR BARCODING | Not Available | 27-
Feb- 15 |
2-
Aug -17 |
23-
Nov -17 |
| US20170336
412 |
Multiplex Immuno Screening Assay | Institut Pasteur | 4-
May- 12 |
19-
Jul- 17 |
23-
Nov -17 |
| US20170336
411 |
B-CELL ANTIGEN PRESENTING CELL ASSAY | The University of Pittsburgh – Of the Commonwealth System of Higher Education | 8-
Apr- 10 |
9-
Aug -17 |
23-
Nov -17 |
| US20170335
408 |
Methods and Systems of Multi-Assay Processing and Analysis | Not Available | 15-
Mar- 16 |
15-
Mar -17 |
23-
Nov -17 |
| US20170335
374 |
METHODS AND COMPOSITIONS FOR IDENTIFICATION OF SOURCE OF MICROBIAL CONTAMINATION IN A SAMPLE | Not Available | 6-
Mar- 12 |
6-
Jul- 17 |
23-
Nov -17 |
| US20170334
984 |
HUMAN MONOCLONAL ANTIBODIES AGAINST INTERLEUKIN 8 (IL-8) | Not Available | 16-
Dec- 02 |
26-
Apr -16 |
23-
Nov -17 |
| US20170334
973 |
NON-HUMAN PRIMATE-DERIVED PAN-EBOLA AND PAN-FILOVIRUS MONOCLONAL ANTIBODIES DIRECTED AGAINST ENVELOPE GLYCOPROTEINS | Not Available | 28-
Oct- 14 |
27-
Oct -15 |
23-
Nov -17 |
| US20170334
941 |
2′,2′-DIHALO NUCLEOSIDE ANALOGS FOR TREATMENT OF THE FLAVIVIRIDAE FAMILY OF VIRUSES AND CANCER | Not Available | 31-
Oct- 14 |
30-
Oct -15 |
23-
Nov -17 |
| US20170334
919 |
2-((4-AMINO-3-(3-FLUORO-5-HYDROXYPHENYL)-1H- PYRAZOLO[3,4-D]PYRIMIDIN-1 -YL)METHYL)-3-(2-(TRIFLUORO-MET HYL)BENZYL)QUINAZOLIN-4(3H)-ONE DERIVATIVES AND THEIR USE AS PHOSPHOINOSITIDE 3-KINASE INHIBITORS | RESPIVERT LTD. | 15-
Mar- 13 |
27-
Jul- 17 |
23-
Nov -17 |
| US20170334
864 |
3,5-DIAMINO-6-CHLORO-N-(N-(4-(4-(2-(HEXYL(2,3,4,5,6-
PENTAHYDROXYHEXYL)AMINO)ETHOXY)PHENYL)BUTYL) CARBAMIMIDOYL)PYRAZINE-2-CARBOXAMIDE |
Parion Sciences, Inc. | 27-
Jun- 11 |
1-
Mar -17 |
23-
Nov -17 |
| US20170333
586 |
ADDITIVE COMPOSITIONS FOR PIGMENTED DISINFECTION AND METHODS THEREOF | Not Available | 8-
Dec- 14 |
23-
May -15 |
23-
Nov -17 |
| US20170333
553 |
LIPIDATED IMMUNE RESPONSE MODIFIER COMPOUND COMPOSITIONS, FORMULATIONS, AND METHODS | Not Available | 21-
May- 13 |
1-
Aug -17 |
23-
Nov -17 |
| US20170333
494 |
PROBIOTIC THERAPEUTIC APPLICATIONS | Not Available | 10-
Nov- 14 |
9-
Nov -15 |
23-
Nov -17 |
| US20170333
457 |
Anti-Viral Azide Containing Compounds | Not Available | 28-
Jul-10 |
8-
Aug -17 |
23-
Nov -17 |
| US20170333
267 |
MOBILE CLINICS | Baylor College of Medicine | 12-
Nov- 14 |
11-
Nov -15 |
23-
Nov -17 |
| US20170328
819 |
SAMPLE FIXATION AND STABILISATION | Not Available | 1-
Mar- 13 |
30-
May -17 |
16-
Nov -17 |
| US20170327
543 |
POLYIONIC PAPILLOMA VIRUS-LIKE PARTICLE (VLP) VACCINES | Not Available | 8-
Sep- 10 |
31-
Jan -17 |
16-
Nov -17 |
| US20170327
472 |
CHLORO-PYRAZINE CARBOXAMIDE DERIVATIVES WITH EPITHELIAL
SODIUM CHANNEL BLOCKING ACTIVITY |
Parion Sciences, Inc. | 17-
Dec- 12 |
7-
Mar -17 |
16-
Nov -17 |
| US20170327
439 |
DIHYDRONAPHTHALENE DERIVATIVE | ONO PHARMACEUTICAL CO., LTD. | 3-
Dec- 14 |
3-
Dec -14 |
16-
Nov -17 |
| US20170326
256 |
RECOMBINANT PROMOTERS AND VECTORS FOR PROTEIN EXPRESSION IN LIVER AND USE THEREOF | Children’s Healthcare of Atlanta, Inc. | 16-
Apr- 15 |
15-
Apr -16 |
16-
Nov -17 |
| US20170326
123 |
Throat solution for treatment of cold, flu and sore throat | Not Available | 12-
May- 16 |
12-
May -16 |
16-
Nov -17 |
| US20170322
682 |
SYSTEM AND METHOD FOR DETECTING, COLLECTING, ANALYZING, AND COMMUNICATING EVENT-RELATED INFORMATION | Georgetown University | 25-
Feb- 08 |
27-
Jul- 17 |
9-
Nov -17 |
| US20170322
201 |
ANTIGEN PRESENTING CELL ASSAY | University of Pittsburgh – Of the Com monwealth System of Higher Education | 8-
Apr- 10 |
20-
Jul- 17 |
9-
Nov -17 |
| US20170321
192 |
Recombinant RNA Viruses and Uses Thereof | Icahn School of Medicine at Mount Sinai | 6-
Jun- 10 |
6-
Apr -17 |
9-
Nov -17 |
| US20170319
712 |
METHODS AND COMPOSITIONS FOR ENHANCING IMMUNE RESPONSE | 3M INNOVATIVE PROPERTIES COMPANY | 10-
Apr- 03 |
27-
Jul- 17 |
9-
Nov -17 |
| US20170319
673 |
TRI-SEGMENTED ARENAVIRUSES AS VACCINE VECTORS | Not Available | 13-
Nov- 14 |
12-
Nov -15 |
9-
Nov -17 |
| US20170319
551 |
NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 9-
May- 12 |
24-
Jan -17 |
9-
Nov -17 |
| US20170313
765 |
DIRECT EXPRESSION OF ANTIBODIES | Not Available | 29-
Oct- 14 |
28-
Oct -15 |
2-
Nov -17 |
| US20170313
685 |
BROAD-SPECTRUM NON-COVALENT CORONAVIRUS PROTEASE INHIBITORS | Purdue Research Foundation | 28-
Apr- 16 |
28-
Apr -17 |
2-
Nov -17 |
| US20170312
371 |
MODIFIED VIRUS-LIKE PARTICLES OF CMV | SAIBA GMBH | 22-
Oct- 14 |
20-
Oct -15 |
2-
Nov -17 |
| US20170312
357 |
MANUFACTURE OF SURFACTANT-CONTAINING COMPOSITIONS | Not Available | 2-
Dec- 14 |
2-
Dec -15 |
2-
Nov -17 |
| US20170308
679 |
BIOSECURITY SCREENING SYSTEM AND METHOD | Not Available | 16-
Oct- 14 |
12-
Oct -15 |
26-
Oct- 17 |
| US20170307
562 |
CHEMICALLY DIFFERENTIATED SENSOR ARRAY | Nanomedical Diagnostics, Inc. | 28-
Apr- 14 |
8-
May -17 |
26-
Oct- 17 |
| US20170306
354 |
ADENO-ASSOCIATED VIRUS (AAV) SEROTYPE 8 SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 17-
Dec- 01 |
1-
May -17 |
26-
Oct- 17 |
| US20170306
293 |
MEANS AND METHODS FOR INFLUENCING THE STABILITY OF ANTIBODY PRODUCING CELLS | Not Available | 9-
Dec- 05 |
2-
Jun -17 |
26-
Oct- 17 |
| US20170306
001 |
CHIMERIZATION AND CHARACTERIZATION OF A MONOCLONAL ANTIBODY WITH POTENT NEUTRALIZING ACTIVITY ACROSS MULTIPLE INFLUENZA A H5N1 CLADES | NATIONAL UNIVERSITY OF SINGAPORE | 27-
Mar- 14 |
27-
Mar -15 |
26-
Oct- 17 |
| US20170305
868 |
ARYLALKYL-AND ARYLOXYALKYL-SUBSTITUTED EPITHELIAL SODIUM CHANNEL BLOCKING COMPOUNDS | Parion Sciences, Inc. | 13-
Dec- 13 |
1-
Mar -17 |
26-
Oct- 17 |
| US20170304
829 |
PRINTED CIRCUIT BOARD HEATER FOR AN AMPLIFICATION MODULE | Click Diagnostics, Inc. | 22-
Apr- 16 |
21-
Apr -17 |
26-
Oct- 17 |
| US20170304
466 |
AAV-Based Gene Therapy | Not Available | 6-
Oct- 14 |
6-
Oct -15 |
26-
Oct- 17 |
| US20170304
459 |
METHODS AND COMPOSITIONS FOR INHALATION DELIVERY OF CONJUGATED OLIGONUCLEOTIDE | Not Available | 10-
Oct- 14 |
7-
Oct -15 |
26-
Oct- 17 |
| US20170304
429 |
VACCINATION OF IMMUNOCOMPROMISED SUBJECTS | Not Available | 26-
Sep- 14 |
25-
Sep -15 |
26-
Oct- 17 |
| US20170304
420 |
POLYMER ADJUVANT | Oxford University Innovation Limited | 10-
Oct- 14 |
9-
Oct -15 |
26-
Oct- 17 |
| US20170304
354 |
TREATMENT OF DISEASE WITH POLY-N-ACETYLGLUCOSAMINE NANOFIBERS | Marine Polymer Technologies, Inc. | 15-
Apr- 11 |
13-
Mar -17 |
26-
Oct- 17 |
| US20170299
591 |
EXOSOME-MEDIATED DIAGNOSIS OF HEPATITIS VIRUS INFECTIONS AND DISEASES | Not Available | 6-
Oct- 08 |
13-
Dec -16 |
19-
Oct- 17 |
| US20170298
100 |
ANTI-VIRAL PEPTIDES | Not Available | 1-
Oct- 14 |
30-
Sep -15 |
19-
Oct- 17 |
| US20170296
663 |
Conjugates of Cell Binding Molecules with Cytotoxic Agents | Hangzhou DAC Biotech Co., Ltd. | 16-
Apr- 14 |
16-
Apr -14 |
19-
Oct- 17 |
| US20170296
574 |
Method of Treating Inflammation | Not Available | 1-
Apr- 10 |
28-
Jun -17 |
19-
Oct- 17 |
| US20170292
132 |
TISSUE PREFERENTIAL CODON MODIFIED EXPRESSION CASSETTES, VECTORS CONTAINING SAME, AND USES THEREOF | Not Available | 29-
Apr- 13 |
20-
Jun -17 |
12-
Oct- 17 |
| US20170290
909 |
METHODS AND COMPOSITIONS FOR INTRA-NASAL IMMUNIZATION WITH RECOMBINANT MVA ENCODING FLAGELLIN | Bavarian Nordic A/S | 26-
Sep- 14 |
25-
Sep -15 |
12-
Oct- 17 |
| US20170281
966 |
Device to Kill Micro-Organisms Inside the Respiratory Tract | Not Available | 1-
Apr- 16 |
28-
Mar -17 |
5-
Oct- 17 |
| US20170281
759 |
BISPHOSPHONATE-CONTAINING VACCINE PHARMACEUTICAL COMPOSITION FOR HUMORAL IMMUNITY | NITTO DENKO CORPORATION | 3-
Sep- 14 |
2-
Sep -15 |
5-
Oct- 17 |
| US20170275
621 |
CHIRAL CONTROL | Not Available | 13-
Jul-12 |
17-
Mar -17 |
28-
Sep -17 |
| US20170275
592 |
CULTURE MEDIUM | Koninklijke Nederlandse Akademie Van Wetenschappen | 27-
Nov- 14 |
27-
Nov -15 |
28-
Sep -17 |
| US20170275
323 |
GLYCOLIPIDS AND PHARMACEUTICAL COMPOSITIONS THEREOF FOR USE IN THERAPY | THE UNIVERSITY OF NOTTINGHAM | 4-
Apr- 14 |
7-
Apr -15 |
28-
Sep -17 |
| US20170275
253 |
BENZAZEPINE SULFONAMIDE COMPOUNDS | Hoffmann-La Roche Inc. | 18-
Dec- 14 |
14-
Jun -17 |
28-
Sep -17 |
| US20170275
243 |
Lipids and Lipid Compositions for the Delivery of Active Agents | Novartis AG | 5-
Sep- 14 |
4-
Sep -15 |
28-
Sep -17 |
| US20170274
064 |
MODIFIED BAT INFLUENZA VIRUSES AND THEIR USES | Not Available | 5-
Sep- 14 |
4-
Sep -15 |
28-
Sep -17 |
| US20170274
024 |
AAV Vectors Targeted to Oligodendrocytes | Not Available | 28-
Sep- 12 |
17-
Apr -17 |
28-
Sep -17 |
| US20170267
969 |
Animal Protein-Free Media for Cultivation of Cells | Baxalta GmbH | 29-
Oct- 04 |
18-
May -17 |
21-
Sep -17 |
| US20170267
722 |
INHIBITORY PEPTIDES OF VIRAL INFECTION | Not Available | 17-
Jul-14 |
25-
May -17 |
21-
Sep -17 |
| US20170267
649 |
STABLE SODIUM CHANNEL BLOCKERS | PARION SCIENCES, INC. | 30-
Jun- 14 |
30-
Jan -17 |
21-
Sep -17 |
| US20170266
272 |
TRANSGENIC VERO-CD4/CCR5 CELL LINE | Not Available | 2-
Oct- 15 |
6-
Apr -17 |
21-
Sep -17 |
| US20170266
190 |
DRUG COMBINATION | Not Available | 15-
Mar- 13 |
5-
Jun -17 |
21-
Sep -17 |
| US20170266
160 |
METHODS FOR TREATING PULMONARY EMPHYSEMA USING SUBSTITUTED 2-AZA-BICYCLO[2.2.1]HEPTANE-3-CARBOXYLIC ACID (BENZYL-CYANO-METHYL)-AMIDES INHIBITORS OF CATHEPSIN C | Not Available | 14-
Mar- 13 |
6-
Jun -17 |
21-
Sep -17 |
| US20170261
431 |
CONTINUOUS PROCESS FOR PERFORMING MULTIPLE NUCLEIC ACID AMPLIFICATION ASSAYS | Not Available | 10-
Mar- 05 |
19-
May -17 |
14-
Sep -17 |
| US20170260
223 |
ENANTIOMERS OF THE 1′,6′-ISOMER OF NEPLANOCIN A | Not Available | 4-
Aug- 14 |
17-
May -17 |
14-
Sep -17 |
| US20170260
147 |
ANTIVIRAL COMPOUNDS AND METHODS | Biotron Limited | 26-
Jun- 03 |
23-
May -17 |
14-
Sep -17 |
| US20170259
976 |
DEGRADABLE MATERIALS AND PACKAGING MADE FROM SAME | MONOSOL, LLC | 3-
Oct- 14 |
2-
Oct -15 |
14-
Sep -17 |
| US20170258
904 |
Antagonism of the VIP Signaling Pathway | Not Available | 2-
Feb- 11 |
22-
May -17 |
14-
Sep -17 |
| US20170258
893 |
MERS-CoV Vaccine | Not Available | 29-
Nov- 13 |
26-
Nov -14 |
14-
Sep -17 |
| US20170253
861 |
HIGHLY EFFICIENT INFLUENZA MATRIX (M1) PROTEINS | Not Available | 11-
Jul-03 |
6-
Oct -16 |
7-
Sep -17 |
| US20170252
430 |
POLYMERIC CARRIER CARGO COMPLEX FOR USE AS AN IMMUNOSTIMULATING AGENT OR AS AN ADJUVANT | Not Available | 1-
Apr- 14 |
1-
Apr -15 |
7-
Sep -17 |
| US20170252
417 |
PROTEIN-CHAPERONED T-CELL VACCINES | Not Available | 7-
Mar- 16 |
7-
Mar -17 |
7-
Sep -17 |
| US20170247
688 |
ENZYMATIC ENCODING METHODS FOR EFFICIENT SYNTHESIS OF LARGE LIBRARIES | NUEVOLUTION A/S | 1-
Dec- 05 |
12-
Dec -16 |
31-
Aug -17 |
| US20170247
453 |
SOLUBLE ENGINEERED MONOMERIC FC | The United States of America, as represented by the Secretary, Department of Health and Human Serv | 16-
Mar- 12 |
9-
May -17 |
31-
Aug -17 |
| US20170247
423 |
STAPLED INTRACELLULAR-TARGETING ANTIMICROBIAL PEPTIDES TO TREAT INFECTION | Not Available | 29-
Feb- 16 |
28-
Feb -17 |
31-
Aug -17 |
| US20170246
347 |
MATERIALS WITH IMPROVED PROPERTIES | Not Available | 1-
Nov- 15 |
5-
May -17 |
31-
Aug -17 |
| US20170241
998 |
METHOD FOR THE IMMOBILIZATION OF BIOMOLECULES | Not Available | 22-
Feb- 16 |
17-
Feb -17 |
24-
Aug -17 |
| US20170240
639 |
ACTRII ANTAGONISTS FOR USE IN INCREASING IMMUNE ACTIVITY | Not Available | 22-
Feb- 16 |
22-
Feb -17 |
24-
Aug -17 |
| US20170239
397 |
MODIFIED ALGINATES FOR ANTI-FIBROTIC MATERIALS AND APPLICATIONS | Not Available | 1-
Nov- 15 |
5-
May -17 |
24-
Aug -17 |
| US20170239
364 |
METHODS AND COMPOSITIONS RELATED TO INHIBITION OF VIRAL ENTRY | Not Available | 28-
Mar- 11 |
2-
Mar -17 |
24-
Aug -17 |
| US20170239
349 |
COMPOSITIONS AND METHODS RELATED TO NEUROLOGICAL DISORDERS | Not Available | 20-
Feb- 15 |
6-
Jun -16 |
24-
Aug -17 |
| US20170239
291 |
METHOD OF PREVENTING OR TREATING SINUSITIS WITH OXIDATIVE REDUCTIVE POTENTIAL WATER SOLUTION | SONOMA PHARMACEUTICALS, INC. | 30-
Dec- 03 |
9-
May -17 |
24-
Aug -17 |
| US20170234
781 |
MEMBRANE-ASSISTED PURIFICATION | Accelerate Diagnostics, Inc. | 7-
Mar- 11 |
3-
May -17 |
17-
Aug -17 |
| US20170226
593 |
HANDHELD NUCLEIC ACID-BASED ASSAY FOR RAPID IDENTIFICATION | Not Available | 8-
Feb- 16 |
8-
Feb -16 |
10-
Aug -17 |
| US20170226
511 |
APTAMERS FOR BINDING FLAVIVIRUS PROTEINS | National University of Singapore | 13-
Nov- 13 |
13-
Nov -14 |
10-
Aug -17 |
| US20170226
232 |
MODIFIED ALGINATES FOR ANTI-FIBROTIC MATERIALS AND APPLICATIONS | Not Available | 1-
Nov- 15 |
2-
Nov -16 |
10-
Aug -17 |
| US20170226
222 |
BISPECIFIC ANTIBODY | Not Available | 20-
Nov- 13 |
18-
Jan -17 |
10-
Aug -17 |
| US20170226
173 |
GM-CSF and IL-4 Conjugates, Compositions, and Methods Related Thereto | Not Available | 23-
Oct- 12 |
19-
Apr -17 |
10-
Aug -17 |
| US20170224
813 |
VACCINE PHARMACEUTICAL COMPOSITION FOR SUPPRESSING APOPTOSIS OF CTL OR INHIBITING SUPPRESSION OF INDUCTION OF CTL | NITTO DENKO CORPORATION | 4-
Aug- 14 |
4-
Aug -15 |
10-
Aug -17 |
| US20170224
812 |
LIQUID IMMUNITY INDUCTION-PROMOTING COMPOSITION AND VACCINE PHARMACEUTICAL COMPOSITION THAT INCLUDE THROMBOSIS TREATMENT DRUG | NITTO DENKO CORPORATION | 4-
Aug- 14 |
4-
Aug -15 |
10-
Aug -17 |
| US20170224
616 |
OIL-IN-WATER EMULSIONS THAT CONTAIN NUCLEIC ACIDS | GLAXOSMITHKLINE BIOLOGICALS SA | 6-Jul-
11 |
24-
Apr -17 |
10-
Aug -17 |
| US20170219
560 |
MALARIA ANTIGEN SCREENING METHOD | United States of America as Represented by the Secretary of the Navy | 31-
Aug- 05 |
10-
Apr -17 |
3-
Aug -17 |
| US20170216
431 |
VACCINE PHARMACEUTICAL COMPOSITION FOR TRANSDERMAL ADMINISTRATION | NITTO DENKO CORPORATION | 2-
Oct- 14 |
1-
Oct -15 |
3-
Aug -17 |
| US20170216
430 |
IMMUNE-INDUCTION-PROMOTING COMPOSITION INCLUDING NUCLEAR RECEPTOR LIGAND, AND VACCINE PHARMACEUTICAL COMPOSITION | NITTO DENKO CORPORATION | 4-
Aug- 14 |
4-
Aug -15 |
3-
Aug -17 |
| US20170216
429 |
COMPOSITION FOR ENHANCING INDUCTION OF HUMORAL IMMUNITY, AND VACCINE PHARMACEUTICAL COMPOSITION | NITTO DENKO CORPORATION | 4-
Aug- 14 |
4-
Aug -15 |
3-
Aug -17 |
| US20170216
427 |
Coronavirus | Not Available | 23-
Jul-14 |
23-
Jul- 15 |
3-
Aug -17 |
| US20170216
348 |
METAL NANOCLUSTERS AND USES THEREOF | Not Available | 7-
Aug- 14 |
7-
Aug -15 |
3-
Aug -17 |
| US20170216
347 |
ANIONICALLY MODIFIED POLYALLYLAMINE DERIVATIVE, USE OF ANIONICALLY MODIFIED POLYALLYLAMINE DERIVATIVE AS MEDICINE, PARTICULARLY FOR PROPYLAXIS AND TREATMENT OF INFECTIONS OF RESPIRATORY TRACT CAUSED BY HUMAN METAPNEUMOVIRUS (HMPV), HUMAN RHINOVIRUSES (HRV), AND INFECTION BY INFLUENZA VIRUS TYPE A (IAV) AND PHARMACEUTICAL COMPOSITION COMPRISING THE ANIONICALLY MODIFIED POLYALLYLAMINE DERIVATIVE | UNIWERSYTET JAGIELLONSKI | 29-
Jul-14 |
29-
Jul- 15 |
3-
Aug -17 |
| US20170212
116 |
BIOSENSORS FOR THE DETECTION OF INFECTION AND ASSOCIATED MALADIES | ULISSE BIOMED SRL | 31-
Jan- 14 |
26-
Jan -15 |
27-
Jul- 17 |
| US20170212
019 |
POLYMER STABILIZATION OF CHROMOGEN SOLUTIONS | Not Available | 23-
Jan- 12 |
10-
Apr -17 |
27-
Jul- 17 |
| US20170211
096 |
LCMV-GP-VSV-Pseudotyped Vectors and Tumor-Infiltrating Virus- Producing Cells for the Therapy of Tumors | Not Available | 29-
Jun- 11 |
7-
Apr -17 |
27-
Jul- 17 |
| US20170211
069 |
USE OF THE CHROMOSOME 19 MICRORNA CLUSTER (C19MC) FOR TREATING MICROBIAL DISEASE AND PROMOTING AUTHOPHAGY | University of Pittsburgh – Of the Com monwealth System of Higher Education | 7-
Mar- 12 |
27-
Jan -17 |
27-
Jul- 17 |
| US20170211
058 |
METHOD AND APPARATUS FOR AUTOMATED PROCESSING OF POOLED SAMPLES | Not Available | 6-
Aug- 14 |
5-
Aug -15 |
27-
Jul- 17 |
| US20170209
844 |
MICROSPOTTING DEVICE | Not Available | 18-
Apr- 12 |
5-
Jan -17 |
27-
Jul- 17 |
| US20170209
595 |
DISULFUR BRIDGE LINKERS FOR CONJUGATION OF A CELLBINDING MOLECULE | Suzhou M-Conj Biotech Co., Ltd. | 15-
Jul-15 |
5-
Apr -17 |
27-
Jul- 17 |
| US20170209
590 |
METHODS AND REAGENTS FOR EFFICIENT AND TARGETED DELIVERY OF THERAPEUTIC MOLECULES TO CXCR4 CELLS | Not Available | 13-
Jan- 11 |
10-
Jan -17 |
27-
Jul- 17 |
| US20170209
570 |
Carbon Nanotube Compositions and Methods of Use Thereof | Not Available | 19-
Mar- 08 |
17-
Mar -17 |
27-
Jul- 17 |
| US20170209
376 |
COMPOSITIONS WITH MODIFIED NUCLEASES TARGETED TO VIRAL NUCLEIC ACIDS AND METHODS OF USE FOR PREVENTION AND TREATMENT OF VIRAL DISEASES | Not Available | 14-
Apr- 04 |
10-
Mar -17 |
27-
Jul- 17 |
| US20170205
399 |
POLYMERS AND CONJUGATES COMPRISING THE SAME | Not Available | 2-
Oct- 14 |
31-
Mar -17 |
20-
Jul- 17 |
| US20170204
143 |
Constrained proteins and uses therefor | Not Available | 21-
Jul-14 |
21-
Jul- 15 |
20-
Jul- 17 |
| US20170204
083 |
THERAPEUTIC HYDROXYPYRIDINONES, HYDROXYPYRIMIDINONES AND HYDROXYPYRIDAZINONES | RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY | 11-
Sep- 12 |
13-
Jan -17 |
20-
Jul- 17 |
| US20170202
975 |
DISULFUR BRIDGE LINKERS FOR CONJUGATION OF A CELLBINDING MOLECULE | Suzhou M-Conj Biotech Co., Ltd. | 15-
Jul-15 |
5-
Apr -17 |
20-
Jul- 17 |
| US20170202
960 |
CATIONIC OIL-IN-WATER EMULSIONS | GLAXOSMITHKLINE BIOLOGICALS, SA | 6-Jul-
11 |
23-
Mar -17 |
20-
Jul- 17 |
| US20170202
959 |
ADJUVANT COMPOSITIONS AND RELATED METHODS | Not Available | 24-
Mar- 15 |
23-
Mar -17 |
20-
Jul- 17 |
| US20170202
956 |
Methods and Compositions for Inhibiting Akt3 | Not Available | 15-
Jan- 16 |
17-
Jan -17 |
20-
Jul- 17 |
| US20170202
955 |
Adjuvanted Influenza Vaccines for Pediatric Use | Not Available | 22-
Feb- 08 |
30-
Dec -16 |
20-
Jul- 17 |
| US20170202
949 |
DEFECTIVE RIBOSOMAL PRODUCTS IN BLEBS (DRIBBLES) AND METHODS OF USE TO STIMULATE AN IMMUNE RESPONSE | Providence Health & Services – Oregon | 29-
Jul-05 |
29-
Dec -16 |
20-
Jul- 17 |
| US20170202
829 |
Specific Akt3 Inhibitor and Uses Thereof Cross-Reference to Related Applications | Not Available | 15-
Jan- 16 |
17-
Jan -17 |
20-
Jul- 17 |
| US20170196
979 |
LIPIDS AND LIPID COMPOSITIONS FOR THE DELIVERY OF ACTIVE AGENTS | Novartis AG | 8-
Mar- 13 |
27-
Sep -16 |
13-
Jul- 17 |
| US20170196
954 |
PRIME-BOOST REGIMENS WITH A TLR4 AGONIST ADJUVANT AND A LENTIVIRAL VECTOR | Not Available | 15-
Jul-14 |
14-
Jul- 15 |
13-
Jul- 17 |
| US20170191
933 |
Systems and Methods for Analyzing a Sample and for Monitoring the Performance of an Optical Signal Detector | Not Available | 31-
Dec- 15 |
23-
Dec -16 |
6-
Jul- 17 |
| US20170191
079 |
METHOD OF INCREASING THE FUNCTION OF AN AAV VECTOR | Not Available | 7-
Apr- 05 |
18-
Jan -17 |
6-
Jul- 17 |
| US20170190
770 |
HUMAN MONOCLONAL ANTIBODIES AGAINST INTERLEUKIN 8 (IL-8) | CORMORANT PHARMACEUTICALS AB | 16-
Dec- 02 |
26-
Apr -16 |
6-
Jul- 17 |
| US20170189
521 |
LIPIDATED IMMUNE RESPONSE MODIFIER COMPOUND COMPOSITIONS, FORMULATIONS, AND METHODS | 3M Innovative Properties Company | 17-
Aug- 10 |
19-
Aug -16 |
6-
Jul- 17 |
| US20170175
140 |
METHODS FOR USING A 5′-EXONUCLEASE TO INCREASE HOMOLOGOUS RECOMBINATION IN EUKARYOTIC CELLS | Not Available | 16-
Dec- 15 |
14-
Dec -16 |
22-
Jun- 17 |
| US20170173
585 |
POINT OF CARE POLYMERASE CHAIN REACTION DEVICE FOR DISEASE DETECTION | Not Available | 11-
Jul-14 |
10-
Jul- 15 |
22-
Jun- 17 |
| US20170173
176 |
ACETYLENEDICARBOXYL LINKERS AND THEIR USES IN SPECIFIC CONJUGATION OF A CELL-BINDING MOLECULE | SUZHOU M-CONJ BIOTECH CO., LTD. | 15-
Jul-15 |
3-
Mar -17 |
22-
Jun- 17 |
| US20170173
168 |
ACETYLENEDICARBOXYL LINKERS AND THEIR USES IN SPECIFIC CONJUGATION OF A CELL-BINDING MOLECULE | SUZHOU M-CONJ BIOTECH CO., LTD. | 15-
Jul-15 |
3-
Mar -17 |
22-
Jun- 17 |
| US20170173
164 |
HYDRAZINO 1H-IMIDAZOQUINOLIN-4-AMINES AND CONJUGATES MADE THEREFROM | Not Available | 3-
Jun- 11 |
6-
Mar -17 |
22-
Jun- 17 |
| US20170168
052 |
EXOSOME-MEDIATED DIAGNOSIS OF HEPATITIS VIRUS INFECTIONS AND DISEASES | Not Available | 6-
Oct- 08 |
13-
Dec -16 |
15-
Jun- 17 |
| US20170168
044 |
QUANTITATIVE ANALYSIS METHOD BASED ON AIR PRESSURE MEASURING | XIAMEN UNIVERSITY | 9-
Jun- 14 |
24-
Sep -14 |
15-
Jun- 17 |
| US20170166
574 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.P.A. | 14-
Dec- 15 |
12-
Dec -16 |
15-
Jun- 17 |
| US20170165
366 |
Anti-TIGIT Antigen-Binding Proteins and Methods of Use Thereof | Not Available | 1-
Oct- 15 |
13-
Feb -17 |
15-
Jun- 17 |
| US20170165
359 |
IMMUNOSTIMULATORY COMBINATIONS OF TLR LIGANDS AND METHODS OF USE | Not Available | 11-
Aug- 10 |
23-
Aug -16 |
15-
Jun- 17 |
| US20170165
341 |
REPLICATION DEFECTIVE ADENOVIRUS VECTOR IN VACCINATION | Not Available | 24-
Aug- 12 |
15-
Feb -17 |
15-
Jun- 17 |
| US20170165
230 |
USE OF GSK-3 INHIBITORS OR ACTIVATORS WHICH MODULATE PD-1 OR T-BET EXPRESSION TO MODULATE T CELL IMMUNITY | Not Available | 9-
Apr- 14 |
9-
Apr -15 |
15-
Jun- 17 |
| US20170160
218 |
APPARATUS AND SYSTEM FOR PERFORMING THERMAL MELT ANALYSES AND AMPLIFICATIONS | Not Available | 31-
Jul-12 |
23-
Feb -17 |
8-
Jun- 17 |
| US20170159
027 |
ADENO-ASSOCIATED VIRUS (AAV) CLADES, SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 30-
Sep- 03 |
15-
Feb -17 |
8-
Jun- 17 |
| US20170159
026 |
Novel Recombinant Adeno-Associated Virus Capsids with Enhanced Human Skeletal Muscle Tropism | Not Available | 2-
Dec- 15 |
2-
Dec -16 |
8-
Jun- 17 |
| US20170158
752 |
MIDDLE EAST RESPIRATORY SYNDROME CORONAVIRUS NEUTRALIZING ANTIBODIES AND METHODS OF USE THEREOF | Not Available | 25-
Apr- 14 |
27-
Apr -15 |
8-
Jun- 17 |
| US20170157
262 |
CONJUGATES OF CELL BINDING MOLECULES WITH CYTOTOXIC AGENTS | Not Available | 12-
Jul-12 |
12-
Jul- 12 |
8-
Jun- 17 |
| US20170157
151 |
Broad Spectrum Antiviral and Methods of Use | Not Available | 17-
Apr- 06 |
16-
Feb -17 |
8-
Jun- 17 |
| US20170152
274 |
CHARGED LINKERS AND THEIR USES FOR CONJUGATION | Hangzhou DAC Biotech Co., Ltd. | 28-
Feb- 14 |
28-
Feb -14 |
1-
Jun- 17 |
| US20170152
271 |
GAK MODULATORS AS ANTIVIRALS | Katholieke Universiteit Leuven | 23-
Jul-14 |
23-
Jul- 15 |
1-
Jun- 17 |
| US20170151
346 |
DISULFUR BRIDGE LINKERS FOR CONJUGATION OF A CELLBINDING MOLECULE | SUZHOU M-CONJ BIOTECH CO., LTD. | 15-
Jul-15 |
13-
Feb -17 |
1-
Jun- 17 |
| US20170151
291 |
SYNERGISTIC BACTERIAL COMPOSITIONS AND METHODS OF PRODUCTION AND USE THEREOF | Not Available | 25-
Nov- 13 |
25-
Nov -14 |
1-
Jun- 17 |
| US20170145
394 |
TRACKING AND MANIPULATING CELLULAR RNA VIA NUCLEAR DELIVERY OF CRISPR/CAS9 | Not Available | 23-
Nov- 15 |
22-
Nov -16 |
25-
May -17 |
| US20170144
984 |
MODULATORS OF THE RELAXIN RECEPTOR 1 | Not Available | 4-
May- 12 |
25-
Aug -16 |
25-
May -17 |
| US20170143
845 |
ACETYLENEDICARBOXYL LINKERS AND THEIR USES IN SPECIFIC CONJUGATION OF A CELL-BINDING MOLECULE | SUZHOU M-CONJ BIOTECH CO., LTD | 15-
Jul-15 |
3-
Feb -17 |
25-
May -17 |
| US20170143
820 |
IMMUNOGENIC COMBINATIONS | GLAXOSMITHKLINE SA | 13-
Jun- 14 |
12-
Jun -15 |
25-
May -17 |
| US20170143
758 |
INHALATION OF NITRIC OXIDE FOR TREATING RESPIRATORY DISEASES | Advanced Inhalation Therapies (AIT) Ltd. | 11-
Sep- 13 |
4-
Jan -17 |
25-
May -17 |
| US20170143
749 |
SUBSTITUTED NUCLEOSIDES, NUCLEOTIDES AND ANALOGS THEREOF | Not Available | 24-
Jun- 14 |
2-
Feb -17 |
25-
May -17 |
| US20170137
527 |
GITR ANTIBODIES AND METHODS OF INDUCING OR ENHANCING AN IMMUNE RESPONSE | Not Available | 25-
Mar- 05 |
7-
Nov -16 |
18-
May -17 |
| US20170137
430 |
Nuclear Transport Modulators And Uses Thereof | Not Available | 29-
Jul-11 |
22-
Jul- 16 |
18-
May -17 |
| US20170136
118 |
NEUTRALIZING MOLECULES TO VIRAL ANTIGENS | Not Available | 28-
Mar- 08 |
8-
Jun -16 |
18-
May -17 |
| US20170136
043 |
THERAPEUTIC USES OF SELECTED PYRROLOPYRIMIDINE COMPOUNDS WITH ANTI-MER TYROSINE KINASE ACTIVITY | The University of North Carolina at Chapel Hill | 11-
Apr- 14 |
30-
Jan -17 |
18-
May -17 |
| US20170131
271 |
ANTIBODY-NANOPARTICLE CONJUGATES AND METHODS FOR MAKING AND USING SUCH CONJUGATES | Not Available | 27-
Apr- 10 |
12-
Sep -16 |
11-
May -17 |
| US20170130
200 |
INTRACELLULAR GENOMIC TRANSPLANT AND METHODS OF THERAPY | Not Available | 31-
Jul-15 |
29-
Jul- 16 |
11-
May -17 |
| US20170129
947 |
REGULATING THE INTERACTION BETWEEN TAM LIGANDS AND LIPID MEMBRANES WITH EXPOSED PHOSPHATIDYL SERINE | Kolltan Pharmaceuticals, Inc. | 25-
Jul-12 |
20-
Sep -16 |
11-
May -17 |
| US20170129
891 |
SUBSTITUTED IMIDAZOQUINOLINES, IMIDAZOPYRIDINES, AND IMIDAZONAPHTHYRIDINES | Not Available | 18-
Jun- 04 |
23-
Jan -17 |
11-
May -17 |
| US20170128
465 |
Pharmaceutical Compositions and Methods | Not Available | 3-
Aug- 15 |
6-
Dec -16 |
11-
May -17 |
| US20170122
853 |
PHOTO-CONTROLLED REMOVAL OF TARGETS IN VITRO AND IN VIVO | THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERV | 8-
Aug- 14 |
7-
Aug -15 |
4-
May -17 |
| US20170121
372 |
NOVEL DEPSIPEPTIDE AND USES THEREOF | Not Available | 3-
Dec- 12 |
15-
Jun -16 |
4-
May -17 |
| US20170119
872 |
SOLUBLE NEEDLE ARRAYS FOR DELIVERY OF INFLUENZA VACCINES | Not Available | 20-
Aug- 10 |
11-
Oct -16 |
4-
May -17 |
| US20170119
871 |
LOW-ADDITIVE INFLUENZA VACCINES | Not Available | 27-
Jun- 07 |
24-
Jun -16 |
4-
May -17 |
| US20170119
820 |
MODIFIED CELLS AND METHODS OF THERAPY | Not Available | 31-
Jul-15 |
29-
Jul- 16 |
4-
May -17 |
| US20170119
786 |
HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS | Not Available | 8-
Mar- 11 |
11-
Nov -16 |
4-
May -17 |
| US20170119
734 |
TETRAZOLONES AS INHIBITORS OF FATTY ACID SYNTHASE | Not Available | 5-
May- 10 |
22-
Apr -16 |
4-
May -17 |
| US20170118
995 |
MINERAL FUNCTIONAL WATER, METHOD FOR PRODUCING THE SAME, AND METHOD FOR CONTROLLING UNICELLULAR ORGANISMS AND/OR VIRUSES | Riken Techno System Co., Ltd. | 17-
Sep- 14 |
6-
Dec -16 |
4-
May -17 |
| US20170114
053 |
HETEROCYCLIC COMPOUNDS AND METHODS OF USE THEREOF | The United States of America, as represented by the Secretary, Department of Health and Human Serv | 12-
Jun- 14 |
12-
Jun -15 |
27-
Apr- 17 |
| US20170112
929 |
VISTA MODULATORS FOR DIAGNOSIS AND TREATMENT OF CANCER | Not Available | 7-
Sep- 12 |
6-
Jun -16 |
27-
Apr- 17 |
| US20170107
254 |
Modified Antimicrobial Peptides | Not Available | 2-
Apr- 14 |
2-
Apr -15 |
20-
Apr- 17 |
| US20170107
195 |
Chemical Compounds | AstraZeneca AB | 18-
Mar- 14 |
17-
Mar -15 |
20-
Apr- 17 |
| US20170106
077 |
INFLUENZA VIRUS VECTORS AND USES THEREFOR | Not Available | 17-
Mar- 14 |
13-
Mar -15 |
20-
Apr- 17 |
| US20170101
459 |
CLEAVAGE AND EXCHANGE OF MAJOR HISTOCOMPATIBILITY COMPLEX LIGANDS EMPLOYING AZOBENZENE-CONTAINING PEPTIDES | Not Available | 6-
Jun- 14 |
5-
Jun -15 |
13-
Apr- 17 |
| US20170101
413 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.p.A. | 12-
Jun- 14 |
12-
Jun -14 |
13-
Apr- 17 |
| US20170100
474 |
METHODS AND COMPOSITIONS FOR LIVE ATTENUATED VIRUSES | Not Available | 6-
Apr- 07 |
22-
Jul- 16 |
13-
Apr- 17 |
| US20170100
385 |
PHARMACEUTICAL COMPOSITIONS COMPRISING DANIRIXIN FOR TREATING INFECTIOUS DISEASES | GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO. 2) LIMITED | 12-
May- 14 |
8-
May -15 |
13-
Apr- 17 |
| US20170096
646 |
Modified Adenovirus Hexon Protein and Uses Thereof | Not Available | 28-
Apr- 06 |
20-
Dec -16 |
6-
Apr- 17 |
| US20170096
455 |
METHODS AND COMPOSITIONS FOR CHIMERIC CORONAVIRUS SPIKE PROTEINS | The University of North Carolina at Chapel Hill | 20-
Mar- 14 |
20-
Mar -15 |
6-
Apr- 17 |
| US20170096
441 |
PHOSPHONATES WITH REDUCED TOXICITY FOR TREATMENT OF VIRAL INFECTIONS | Not Available | 14-
Apr- 10 |
9-
Sep -16 |
6-
Apr- 17 |
| US20170096
417 |
Multicyclic Compounds And Methods Of Using Same | Karyopharm Therapeutics Inc. | 20-
Sep- 13 |
19-
Sep -14 |
6-
Apr- 17 |
| US20170095
818 |
INTEGRATED MICROFLUIDIC DEVICE FOR TARGET AMPLIFICATION AND MICROARRAY DETECTION | CapitalBio Corporation | 31-
Mar- 14 |
31-
Mar -15 |
6-
Apr- 17 |
| US20170095
521 |
Novel Polygonum Cuspidatum Extracts and Their Use as Photodynamic Inactivating Agents | Not Available | 1-
Oct- 15 |
30-
Sep -16 |
6-
Apr- 17 |
| US20170089
911 |
QUINONE METHIDE ANALOG SIGNAL AMPLIFICATION | Not Available | 24-
Feb- 14 |
24-
Aug -16 |
30-
Mar -17 |
| US20170088
858 |
ADENO-ASSOCIATED VIRUS (AAV) SEROTYPE 8 SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 17-
Dec- 01 |
20-
Oct -16 |
30-
Mar -17 |
| US20170088
848 |
Recombinant Influenza Virus-Like Particles (VLPs) Produced in Transgenic Plants Expressing Hemagglutinin | Medicago Inc. | 21-
Jan- 08 |
2-
Sep -16 |
30-
Mar -17 |
| US20170088
559 |
ALKYLOXY SUBSTITUTED THIAZOLOQUINOLINES AND THIAZOLONAPHTHYRIDINES | Not Available | 9-
Feb- 05 |
12-
Dec -16 |
30-
Mar -17 |
| US20170086
463 |
ANTIVIRAL AGENT | Not Available | 3-
Sep- 08 |
12-
Dec -16 |
30-
Mar -17 |
| US20170082
608 |
Assay for Detecting TH1 and TH2 Cell Populations | Not Available | 16-
May- 14 |
15-
May -15 |
23-
Mar -17 |
| US20170082
607 |
MALARIA ANTIGEN SCREENING METHOD | Not Available | 31-
Aug- 05 |
19-
Apr -13 |
23-
Mar -17 |
| US20170081
655 |
PURIFICATION OF NUCLEIC ACIDS USING COPPER-TITANIUM OXIDES | Not Available | 14-
Jul-15 |
13-
Jul- 16 |
23-
Mar -17 |
| US20170081
393 |
ENGINEERED ANTIBODY CONSTANT DOMAIN MOLECULES | The U.S.A., as represented by the Secretary, Department of Health and Human Services | 31-
Jan- 08 |
2-
Dec -16 |
23-
Mar -17 |
| US20170081
392 |
COMPOSITIONS COMPRISING AAV EXPRESSING DUAL ANTIBODY CONSTRUCTS AND USES THEREOF | Not Available | 13-
May- 14 |
13-
May -15 |
23-
Mar -17 |
| US20170080
084 |
OIL/SURFACTANT MIXTURES FOR SELF-EMULSIFICATION | Not Available | 17-
Mar- 14 |
17-
Mar -15 |
23-
Mar -17 |
| US20170080
079 |
METHODS OF MAKING AND USING LIVE ATTENUATED VIRUSES | Not Available | 23-
Sep- 15 |
23-
Sep -16 |
23-
Mar -17 |
| US20170080
078 |
COMPOSITIONS AND METHODS FOR TREATING AND PREVENTING PORCINE REPRODUCTIVE AND RESPIRATORY SYNDROME | Not Available | 24-
Apr- 12 |
3-
Oct -16 |
23-
Mar -17 |
| US20170079
920 |
Technology for the Preparation of Microparticles | Not Available | 24-
Jul-07 |
5-
Dec -16 |
23-
Mar -17 |
| US20170079
916 |
COMPOSITIONS AND METHODS FOR MODIFIED DENDRIMER NANOPARTICLE DELIVERY | Not Available | 23-
Sep- 15 |
23-
Sep -16 |
23-
Mar -17 |
| US20170079
253 |
TRANSGENIC MICE HAVING A HUMAN MAJOR HISTOCOMPATIBILITY COMPLEX (MHC) PHENOTYPE, EXPERIMENTAL USES AND APPLICATIONS | INSTITUT PASTEUR | 30-
Jul-03 |
17-
Jul- 12 |
23-
Mar -17 |
| US20170073
738 |
Compositions and Methods for Detecting and Quantifying Nucleic Acid Sequences in Blood Samples | Longhorn Vaccines and Diagnostics, LLC | 1-
Oct- 07 |
25-
Nov -16 |
16-
Mar -17 |
| US20170073
730 |
METHODS AND COMPOSITIONS FOR LIBRARY NORMALIZATION | Not Available | 11-
Sep- 15 |
8-
Sep -16 |
16-
Mar -17 |
| US20170073
727 |
METHODS FOR DIAGNOSING INFECTIOUS DISEASES USING ADSORPTION MEDIA | Not Available | 8-
Nov- 13 |
2-
Nov -16 |
16-
Mar -17 |
| US20170073
390 |
Method for Identifying and Validating Dominant T Helper Cell Epitopes Using an HLA-DM-Assisted Class II Binding Assay | Not Available | 6-
Jan- 06 |
5-
Dec -14 |
16-
Mar -17 |
| US20170073
352 |
NOVEL SUBSTITUTED SPIROCYCLES | Not Available | 12-
Sep- 14 |
29-
Nov -16 |
16-
Mar -17 |
| US20170072
053 |
METHODS FOR PREPARING SQUALENE | Not Available | 12-
May- 10 |
22-
Nov -16 |
16-
Mar -17 |
| US20170071
980 |
METHOD OF USING OXIDATIVE REDUCTIVE POTENTIAL WATER SOLUTION IN DENTAL APPLICATIONS | OCULUS INNOVATIVE SCIENCES, INC. | 2-
May- 05 |
22-
Nov -16 |
16-
Mar -17 |
| US20170071
964 |
METHODS FOR TREATING ARENAVIRIDAE AND CORONAVIRIDAE VIRUS INFECTIONS | Not Available | 16-
Sep- 15 |
16-
Sep -16 |
16-
Mar -17 |
| US20170071
867 |
NOVEL NANOPARTICLE COMPOSITIONS | Not Available | 3-
Apr- 13 |
28-
Nov -16 |
16-
Mar -17 |
| US20170067
030 |
ATTENUATED VIRUSES USEFUL FOR VACCINES | Not Available | 30-
Mar- 07 |
7-
Sep -16 |
9-
Mar -17 |
| US20170067
021 |
MODIFIED CELLS AND METHODS OF THERAPY | Not Available | 31-
Jul-15 |
2-
Sep -16 |
9-
Mar -17 |
| US20170066
788 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 16-
Feb- 05 |
18-
Nov -16 |
9-
Mar -17 |
| US20170066
787 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 16-
Feb- 05 |
18-
Nov -16 |
9-
Mar -17 |
| US20170066
731 |
SMALL MOLECULE FATTY ACID SYNTHASE INHIBITORS | Not Available | 7-
Mar- 14 |
5-
Mar -15 |
9-
Mar -17 |
| US20170065
706 |
METHODS AND COMPOSITIONS FOR PRODUCING AN ADENOVIRUS VECTOR FOR USE WITH MULTIPLE VACCINATIONS | Not Available | 2-Jul-
07 |
14-
Sep -16 |
9-
Mar -17 |
| US20170065
693 |
Sequential administration of a replication defective adenovirus vector in vaccination protocols | Not Available | 2-Jul-
07 |
14-
Sep -16 |
9-
Mar -17 |
| US20170065
677 |
EV576 FOR USE IN THE TREATMENT OF VIRAL INFECTIONS OF THE RESPIRATORY TRACT | Volution Immuno Pharmaceuticals SA | 8-
Jan- 10 |
22-
Jul- 16 |
9-
Mar -17 |
| US20170065
636 |
MODIFIED CELLS AND METHODS OF THERAPY | Not Available | 31-
Jul-15 |
29-
Aug -16 |
9-
Mar -17 |
| US20170058
430 |
BACTERIAL IDENTIFICATION IN CLINICAL INFECTIONS | Not Available | 18-
Feb- 14 |
18-
Feb -15 |
2-
Mar -17 |
| US20170058
365 |
SYSTEMS AND METHODS FOR ANALYZING VIRAL NUCLEIC ACIDS | Not Available | 1-
Sep- 15 |
3-
Feb -16 |
2-
Mar -17 |
| US20170057
978 |
ANTI-VIRAL COMPOUNDS, PHARMACEUTICAL COMPOSITIONS, AND METHODS OF USE THEREOF | Kineta, Inc. | 9-
May- 14 |
8-
May -15 |
2-
Mar -17 |
| US20170057
968 |
COMPOUNDS AND COMPOSITIONS AS TOLL-LIKE RECEPTOR 7 AGONISTS | NOVARTIS AG | 1-
May- 14 |
29-
Apr -15 |
2-
Mar -17 |
| US20170057
938 |
CERTAIN (2S)-N-[(1S)-1-CYANO-2-PHENYLETHYL]-1,4- OXAZEPANE-2-CARBOXAMIDES AS DIPEPTIDYL PEPTIDASE 1 INHIBITORS | Not Available | 24-
Jan- 14 |
8-
Nov -16 |
2-
Mar -17 |
| US20170052
190 |
Covalently Linked Thermostable Kinase for Decontamination Process Validation | Not Available | 20-
Feb- 08 |
8-
Jul- 16 |
23-
Feb -17 |
| US20170051
053 |
METHOD OF PROVIDING MONOCLONAL AUTO-ANTIBODIES WITH DESIRED SPECIFICITY | Not Available | 28-
Dec- 11 |
7-
Sep -16 |
23-
Feb -17 |
| US20170051
046 |
H1N1 FLU VIRUS NEUTRALIZING ANTIBODIES | MEDIGEN BIOTECHNOLOGY CORPORATION | 20-
Aug- 15 |
20-
Aug -15 |
23-
Feb -17 |
| US20170051
022 |
ADENOVIRUS COMPRISING AN ALBUMIN-BINDING MOIETY | Not Available | 30-
Apr- 14 |
30-
Apr -15 |
23-
Feb -17 |
| US20170051
007 |
PHARMACEUTICAL COMPOSITIONS AND METHODS | Not Available | 3-
Aug- 15 |
28-
Jul- 16 |
23-
Feb -17 |
| US20170049
813 |
ANTIMICROBIAL SOLUTIONS CONTAINING DICHLORINE MONOXIDE AND METHODS OF MAKING AND USING THE SAME | OCULUS INNOVATIVE SCIENCES, INC. | 13-
Mar- 07 |
8-
Nov -16 |
23-
Feb -17 |
| US20170044
603 |
CAPTURE PRIMERS AND CAPTURE SEQUENCE LINKED SOLID SUPPORTS FOR MOLECULAR DIAGNOSTIC TESTS | Not Available | 31-
Jul-09 |
15-
Aug -16 |
16-
Feb -17 |
| US20170044
595 |
PCR Ready Compositions and Methods for Detecting and Identifying Nucleic Acid Sequences | Longhorn Vaccines and Diagnostics, LLC | 24-
Aug- 07 |
26-
Oct -16 |
16-
Feb -17 |
| US20170044
237 |
ANTIBODIES AND PROCESSES FOR PREPARING THE SAME | Not Available | 16-
May- 08 |
23-
Aug -16 |
16-
Feb -17 |
| US20170044
168 |
COMPOUNDS AND COMPOSITIONS AS TOLL-LIKE RECEPTOR 7 AGONISTS | NOVARTIS AG | 1-
May- 14 |
29-
Apr -15 |
16-
Feb -17 |
| US20170042
994 |
COMPOSITIONS HAVING MEANS FOR TARGETING AT LEAST ONE ANTIGEN TO DENDRITIC CELLS | ASSISTANCE PUBLIQUE – HOPITAUX DE PARIS | 22-
Jul-11 |
29-
Jul- 16 |
16-
Feb -17 |
| US20170042
898 |
METHODS AND COMPOSITIONS FOR TREATING VIRAL OR VIRALLY- INDUCED CONDITIONS | HEMAQUEST PHARMACEUTICALS, INC. | 11-
Mar- 10 |
27-
Oct -16 |
16-
Feb -17 |
| US20170039
316 |
Compositions, processes and algorithms for microbial detection | Not Available | 12-
Nov- 03 |
25-
Oct -04 |
9-
Feb -17 |
| US20170039
314 |
BIOINFORMATIC PROCESSES FOR DETERMINATION OF PEPTIDE BINDING | IOGENETICS, LLC | 23-
Mar- 10 |
13-
Sep -12 |
9-
Feb -17 |
| US20170038
085 |
AIR CURTAIN DEVICE | Not Available | 7-
Aug- 15 |
19-
Nov -15 |
9-
Feb -17 |
| US20170037
457 |
NUCLEIC ACID DETECTION OR QUANTIFICATION METHOD USING MASK OLIGONUCLEOTIDE, AND DEVICE FOR SAME | Not Available | 5-
Feb- 14 |
29-
Jan -15 |
9-
Feb -17 |
| US20170037
379 |
CHIMERIC VIRUSES PRESENTING NON-NATIVE SURFACE PROTEINS AND USES THEREOF | Icahn School of Medicine at Mount Sinai | 2-
Dec- 05 |
3-
Mar -16 |
9-
Feb -17 |
| US20170037
376 |
METHOD FOR PREPARING INDUCED PLURIPOTENT STEM CELL, COMPOSITION USED IN METHOD, AND USES THEREOF | GUANGZHOU INSTITUTES OF BIOMEDICINE AND HEALTH, CHINESE ACADEMY OF SCIENCES | 15-
Nov- 13 |
12-
Nov -14 |
9-
Feb -17 |
| US20170037
090 |
RECOMBINANT EXPRESSION OF MULTIPROTEIN COMPLEXES USING POLYGENES | Not Available | 8-
Nov- 05 |
26-
Oct -16 |
9-
Feb -17 |
| US20170037
045 |
HETEROBIFUNCTIONAL LINKERS WITH POLYETHYLENE GLYCOL SEGMENTS AND IMMUNE RESPONSE MODIFIER CONJUGATES MADE THEREFROM | 3M Innovative Properties Company | 3-
Jun- 11 |
24-
Oct -16 |
9-
Feb -17 |
| US20170035
878 |
Multi-Functional Mucosal Vaccine Platform | Not Available | 11-
Feb- 14 |
11-
Feb -15 |
9-
Feb -17 |
| US20170035
845 |
Compositions and Methods for Inhibiting Pro-Inflammatory Cytokine Gene Expression | Not Available | 7-
Aug- 15 |
5-
Aug -16 |
9-
Feb -17 |
| US20170029
877 |
POLYTAG PROBES | Not Available | 26-
Feb- 10 |
31-
Mar -16 |
2-
Feb -17 |
| US20170029
847 |
ARTIFICIAL NUCLEIC ACID MOLECULES | CureVac AG | 30-
Dec- 13 |
28-
Jun -16 |
2-
Feb -17 |
| US20170029
489 |
HUMAN MONOCLONAL ANTIBODY WITH SPECIFICITY FOR DENGUE VIRUS SEROTYPE 1 E PROTEIN AND USES THEREOF | Not Available | 14-
Dec- 10 |
1-
Jun -16 |
2-
Feb -17 |
| US20170028
082 |
ADENO-ASSOCIATED VIRUS (AAV) CLADES, SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 30-
Sep- 03 |
3-
Aug -16 |
2-
Feb -17 |
| US20170028
054 |
VACCINE COMPOSITION | NITTO DENKO CORPORATION | 4-
Apr- 12 |
11-
Oct -16 |
2-
Feb -17 |
| US20170027
975 |
Methods of Treating Coronavirus Infection | United States Government as represented by the Secretary, Department of Health and Human Services | 7-
Apr- 14 |
7-
Apr -15 |
2-
Feb -17 |
| US20170027
944 |
METHODS FOR TREATING VIRAL DISORDERS | Not Available | 24-
Sep- 09 |
18-
Feb -15 |
2-
Feb -17 |
| US20170022
577 |
METHODS OF TESTING FOR INTRACELLULAR PATHOGENS | Novartis AG | 8-
Mar- 10 |
23-
Sep -16 |
26-
Jan- 17 |
| US20170022
242 |
NOVEL ANTIVIRAL AND ANTITUMORAL COMPOUNDS | KATHOLIEKE UNIVERSITEIT LEUVEN, KU LEUVEN R&D | 17-
Apr- 14 |
17-
Apr -15 |
26-
Jan- 17 |
| US20170021
013 |
D-AMINO ACID DERIVATIVE-MODIFIED PEPTIDOGLYCAN AND METHODS OF USE THEREOF | Not Available | 30-
Nov- 12 |
31-
Mar -16 |
26-
Jan- 17 |
| US20170020
926 |
METHODS AND COMPOSITIONS FOR IMMUNOMODULATION | Not Available | 1-
Apr- 14 |
13-
Mar -15 |
26-
Jan- 17 |
| US20170016
048 |
COMPOSITIONS AND METHODS FOR ENRICHING POPULATIONS OF NUCLEIC ACIDS | Not Available | 18-
May- 15 |
17-
May -16 |
19-
Jan- 17 |
| US20170015
716 |
STABILIZED ANTI-MICROBIAL PEPTIDES | Not Available | 2-Jul-
15 |
1-
Jul- 16 |
19-
Jan- 17 |
| US20170014
496 |
COMBINATION OF VACCINATION AND OX40 AGONISTS | Not Available | 12-
Mar- 14 |
12-
Mar -14 |
19-
Jan- 17 |
| US20170014
423 |
Benzazepine Dicarboxamide Compounds | Hoffmann-La Roche Inc. | 6-
Mar- 15 |
28-
Sep -16 |
19-
Jan- 17 |
| US20170011
131 |
SYSTEM AND METHOD FOR DETECTING, COLLECTING, ANALYZING, AND COMMUNICATING EVENT RELATED INFORMATION | Georgetown University | 25-
Feb- 08 |
23-
Sep -16 |
12-
Jan- 17 |
| US20170010
264 |
EXOSOME-MEDIATED DIAGNOSIS OF HEPATITIS VIRUS INFECTIONS AND DISEASES | Not Available | 6-
Oct- 08 |
20-
Sep -16 |
12-
Jan- 17 |
| US20170009
237 |
Short Interfering RNA (siRNA) Analogues | Not Available | 21-
Mar- 03 |
28-
Mar -16 |
12-
Jan- 17 |
| US20170007
577 |
METHOD OF TREATING OR INHIBITING THE DEVELOPMENT OF BRAIN INFLAMMATION AND SEPSIS | Not Available | 29-
Nov- 02 |
19-
Sep -16 |
12-
Jan- 17 |
| US20170002
042 |
PEPTIDOMIMETIC MACROCYCLES | Not Available | 1-Jul-
15 |
1-
Jul- 16 |
5-
Jan- 17 |
| US20170000
878 |
CONSTRAINED IMMUNOGENIC COMPOSITIONS AND USES THEREFOR | Not Available | 23-
Jun- 10 |
9-
Aug -16 |
5-
Jan- 17 |
| US20170000
873 |
Dimethyl Fumarate and Vaccination Regimens | Not Available | 14-
Mar- 14 |
13-
Mar -15 |
5-
Jan- 17 |
| US20160376
596 |
COMPOSITIONS AND METHODS FOR INDUCING AN ENHANCED IMMUNE RESPONSE USING POXVIRUS VECTORS | Bavarian Nordic A/S | 28-
Nov- 13 |
25-
Nov -14 |
29-
Dec -16 |
| US20160376
321 |
A NOVEL SARS IMMUNOGENIC COMPOSITION | BAYLOR COLLEGE OF MEDICINE | 26-
Nov- 13 |
21-
Nov -14 |
29-
Dec -16 |
| US20160375
137 |
TARGETING LIPIDS | Tekmira Pharmaceuticals Corporation | 4-
Dec- 07 |
22-
Oct -13 |
29-
Dec -16 |
| US20160375
132 |
HOMOGENOUS SUSPENSION OF IMMUNOPOTENTIATING COMPOUNDS AND USES THEREOF | Not Available | 15-
Dec- 09 |
5-
Jul- 16 |
29-
Dec -16 |
| US20160369
268 |
TRANSCRIPTION ACTIVATOR-LIKE EFFECTOR (TALE) LIBRARIES AND METHODS OF SYNTHESIS AND USE | The Board of Regents of the University of Texas System | 1-Jul-
13 |
25-
Jun -14 |
22-
Dec -16 |
| US20160368
956 |
METHOD OF PREVENTIVELY TREATING A SUBJECT AT THE RISK OF DEVELOPING INFECTIONS OF A RESPIRATORY VIRUS | Not Available | 9-
May- 13 |
23-
Aug -16 |
22-
Dec -16 |
| US20160368
904 |
SUBSTITUTED BENZOFURANYL AND BENZOXAZOLYL COMPOUNDS AND USES THEREOF | Not Available | 3-Jul-
13 |
3-
Jul- 14 |
22-
Dec -16 |
| US20160367
587 |
Systemic In Vivo Delivery of Oligonucleotides | OncoImmunin, Inc. | 12-
Jun- 13 |
12-
Jun -14 |
22-
Dec -16 |
| US20160367
188 |
ORAL SENSOR ALERTING AND COMMUNICATION SYSTEM AND DEVELOPERS’ TOOL KIT | Not Available | 17-
Jun- 15 |
10-
Sep -15 |
22-
Dec -16 |
| US20160363
557 |
AMPEROMETRIC GAS SENSOR | Not Available | 25-
Jun- 12 |
25-
Aug -16 |
15-
Dec -16 |
| US20160362
730 |
PHOTO-SELECTIVE METHOD FOR BIOLOGICAL SAMPLE ANALYSIS | Not Available | 26-
Feb- 14 |
26-
Aug -16 |
15-
Dec -16 |
| US20160362
454 |
Identification and Attenuation of the Immunosuppressive Domains in Fusion Proteins of Enveloped RNA Viruses | Not Available | 7-
Oct- 11 |
10-
Jun -16 |
15-
Dec -16 |
| US20160361
382 |
MICROBICIDAL COMPOSITIONS AND METHODS FOR TREATMENT OF VIRAL INFECTIONS | Not Available | 11-
Jun- 15 |
13-
Jun -16 |
15-
Dec -16 |
| US20160361
259 |
Methods for the Preparation of Liposomes | Not Available | 23-
Sep- 09 |
2-
Aug -16 |
15-
Dec -16 |
| US20160354
451 |
METHOD OF PROVIDING PATIENT SPECIFIC IMMUNE RESPONSE IN AMYLOIDOSES AND PROTEIN AGGREGATION DISORDERS | Not Available | 31-
Aug- 07 |
9-
Jun -16 |
8-
Dec -16 |
| US20160354
428 |
METHODS AND COMPOSITIONS RELATED TO INHIBITION OF VIRAL ENTRY | Not Available | 8-
Feb- 07 |
2-
Jun -16 |
8-
Dec -16 |
| US20160354
347 |
Nuclear Transport Modulators and Uses Thereof | Not Available | 9-
May- 12 |
6-
Jan -16 |
8-
Dec -16 |
| US20160348
153 |
EXTRACTION AND PRESERVATION OF NUCLEIC ACID MOLECULES FROM PATHOGENS | THE UNITED STATES OF AMERICA, as represented by the Secretary, Department of Health and Human Serv | 29-
May- 15 |
31-
May -16 |
1-
Dec -16 |
| US20160348
132 |
TC-83-DERIVED ALPHAVIRUS VECTORS, PARTICLES AND METHODS | Not Available | 18-
May- 04 |
11-
Aug -16 |
1-
Dec -16 |
| US20160348
115 |
CpG Oligonucleotide Analogs Containing Hydrophobic T Analogs with Enhanced Immunostimulatory Activity | Not Available | 27-
Sep- 06 |
26-
May -16 |
1-
Dec -16 |
| US20160348
110 |
NUCLEIC ACID CHEMICAL MODIFICATIONS | Not Available | 2-
Mar- 09 |
11-
Aug -16 |
1-
Dec -16 |
| US20160347
816 |
POLYPEPTIDES AND POLYNUCLEOTIDES, AND USES THEREOF FOR TREATMENT OF IMMUNE RELATED DISORDERS AND CANCER | Not Available | 15-
Apr- 11 |
29-
Feb -16 |
1-
Dec -16 |
| US20160347
814 |
VSTM5 POLYPEPTIDES AND USES THEREOF AS A DRUG FOR TREATMENT OF CANCER, INFECTIOUS DISEASES AND IMMUNE RELATED DISEASES | Not Available | 11-
Sep- 13 |
10-
Mar -16 |
1-
Dec -16 |
| US20160347
784 |
NOVEL NUCLEIC ACID PRODRUGS AND METHODS OF USE THEREOF | Not Available | 6-Jul-
09 |
27-
May -16 |
1-
Dec -16 |
| US20160346
309 |
TREATMENT OF VIRAL INFECTIONS BY MODULATION OF HOST CELL METABOLIC PATHWAYS | Not Available | 1-
Jun- 07 |
21-
Dec -15 |
1-
Dec -16 |
| US20160340
713 |
STABILIZING COMPOSITIONS AND METHODS FOR EXTRACTION OF RIBONUCLEIC ACID | Not Available | 6-
Oct- 06 |
20-
May -16 |
24-
Nov -16 |
| US20160340
319 |
SUBSTITUTED BICYCLIC DIHYDROPYRIMIDINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 6-
Feb- 13 |
5-
Aug -16 |
24-
Nov -16 |
| US20160339
097 |
CORONAVIRUS PROTEINS AND ANTIGENS | MJ Biologics, Inc. | 7-
Feb- 14 |
4-
Aug -16 |
24-
Nov -16 |
| US20160338
998 |
HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS AND COMBINATIONS THEREOF | 3-V Biosciences, Inc. | 20-
Dec- 13 |
19-
Dec -14 |
24-
Nov -16 |
| US20160333
356 |
OLIGONUCLEOTIDE MODULATORS OF THE TOLL-LIKE RECEPTOR PATHWAY | Not Available | 3-
Mar- 11 |
28-
Jul- 16 |
17-
Nov -16 |
| US20160333
089 |
Antigenic GM-CSF Peptides and Antibodies to GM-CSF | Not Available | 8-
Feb- 06 |
18-
Jul- 16 |
17-
Nov -16 |
| US20160333
076 |
NEUTRALIZING GP41 ANTIBODIES AND THEIR USE | The United States of America, as represented by the Secretary, Department of Health and Human Serv | 7-
Nov- 11 |
2-
Aug -16 |
17-
Nov -16 |
| US20160331
828 |
NUCLEIC ACID VACCINES | Moderna Therapeutics, Inc. | 23-
Apr- 14 |
5-
Apr -16 |
17-
Nov -16 |
| US20160331
816 |
ORAL DELIVERY OF ANGIOTENSIN CONVERTING ENZYME 2 (ACE2) OR ANGIOTENSIN-(1-7) BIOENCAPSULATED IN PLANT CELLS ATTENUATES PULMONARY HYPERTENSION, CARDIAC DYSFUNCTION AND DEVELOPMENT OF AUTOIMMUNE AND EXPERIMENTALLY INDUCED OCULAR DISORDERS | Not Available | 18-
Oct- 13 |
18-
Apr -16 |
17-
Nov -16 |
| US20160331
758 |
TOLL-LIKE RECEPTOR AGONIST FORMULATIONS AND THEIR USE | Not Available | 1-
Aug- 08 |
21-
Dec -15 |
17-
Nov -16 |
| US20160327
506 |
CAPACITIVE LIQUID CRYSTAL BIOSENSORS | Not Available | 6-
May- 15 |
5-
May -16 |
10-
Nov -16 |
| US20160327
484 |
A METHOD OF PREDICTING A PERFORMANCE CHARACTERISTIC OF A PLANT OR YEAST HYDROLYSATE AND ITS USE | Not Available | 30-
Dec- 13 |
18-
Dec -14 |
10-
Nov -16 |
| US20160326
598 |
METHODS AND COMPOSITIONS FOR PROSTATE CANCER METASTASIS | Not Available | 25-
Mar- 11 |
27-
May -16 |
10-
Nov -16 |
| US20160326
325 |
POWDERED POUCH AND METHOD OF MAKING SAME | Not Available | 16-
Apr- 12 |
19-
Jul- 16 |
10-
Nov -16 |
| US20160326
233 |
NEUTRALIZING HUMAN MONOCLONAL ANTIBODIES AGAINST HEPATITIS B VIRUS SURFACE ANTIGEN | Not Available | 16-
Jan- 14 |
15-
Jan -15 |
10-
Nov -16 |
| US20160326
141 |
HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS FOR USE AGAINST CANCER AND VIRAL INFECTIONS | Not Available | 7-
Jan- 14 |
7-
Jan -15 |
10-
Nov -16 |
| US20160325
283 |
BIOAGENT DETECTION SYSTEMS, DEVICES, AND METHODS | Not Available | 30-
Mar- 09 |
18-
Jul- 16 |
10-
Nov -16 |
| US20160324
834 |
Use of mTOR Inhibitors to Enhance T Cell Immune Responses | Not Available | 5-
Aug- 08 |
16-
May -16 |
10-
Nov -16 |
| US20160320
390 |
COMPOSITIONS AND METHODS FOR CAPTURING EXOSOMES | Not Available | 1-
May- 15 |
1-
May -15 |
3-
Nov -16 |
| US20160318
985 |
Chimeric Virus-Like Particles Incorporating Fusion GPI Anchored GM-CSF and IL-4 Conjugates | Not Available | 29-
Apr- 15 |
29-
Apr -16 |
3-
Nov -16 |
| US20160318
861 |
NOVEL PRODRUGS OF DITHIOL MUCOLYTIC AGENTS | PARION SCIENCES, INC. | 30-
Apr- 15 |
2-
May -16 |
3-
Nov -16 |
| US20160317
647 |
NUCLEIC ACID VACCINES | Moderna Therapeutics, Inc. | 23-
Apr- 14 |
1-
Apr -16 |
3-
Nov -16 |
| US20160317
637 |
IMMUNOMODULATORY COMPOSITIONS AND METHODS OF USE THEREOF | Not Available | 7-
Jan- 14 |
7-
Jan -15 |
3-
Nov -16 |
| US20160317
496 |
METHODS OF TREATING CANCER AND OTHER DISORDERS | Not Available | 12-
Nov- 10 |
27-
Jun -16 |
3-
Nov -16 |
| US20160317
458 |
Lipids and Lipid Compositions for the Delivery of Active Agents | Not Available | 19-
Dec- 13 |
17-
Dec -14 |
3-
Nov -16 |
| US20160312
276 |
METHODS AND COMPOSITIONS FOR WHOLE TRANSCRIPTOME AMPLIFICATION | Not Available | 23-
Apr- 15 |
21-
Apr -16 |
27-
Oct- 16 |
| US20160311
886 |
Constructs Binding to Phosphatidylserine and Their Use in Disease Treatment and Imaging | Not Available | 24-
Jan- 05 |
12-
Jul- 16 |
27-
Oct- 16 |
| US20160311
835 |
NOVEL ANTIBIOTICS | Not Available | 26-
May- 11 |
30-
Nov -15 |
27-
Oct- 16 |
| US20160311
816 |
COMPOSITIONS AND METHODS FOR INHIBITING KINASES | Not Available | 23-
Apr- 15 |
22-
Apr -16 |
27-
Oct- 16 |
| US20160311
759 |
Lipids and Lipid Compositions for the Delivery of Active Agents | Not Available | 19-
Dec- 13 |
17-
Dec -14 |
27-
Oct- 16 |
| US20160310
511 |
Myxovirus Therapeutics, Compounds, and Uses Related Thereto | Not Available | 24-
Oct- 11 |
7-
Jul- 16 |
27-
Oct- 16 |
| US20160305
936 |
DIRECT CLONE ANALYSIS AND SELECTION TECHNOLOGY | Not Available | 13-
Jul-10 |
18-
Feb -16 |
20-
Oct- 16 |
| US20160304
954 |
COMPOSITIONS AND METHODS FOR DETECTING RARE SEQUENCE VARIANTS | Not Available | 11-
Dec- 13 |
11-
Dec -14 |
20-
Oct- 16 |
| US20160304
942 |
Enhanced Methods of Ribonucleic Acid Hybridization | Not Available | 6-
Dec- 13 |
5-
Dec -14 |
20-
Oct- 16 |
| US20160304
904 |
Directed Evolution and In Vivo Panning of Virus Vectors | Not Available | CO 1 1 | 28-
Jun -16 |
20-
Oct- 16 |
| US20160304
883 |
ARTIFICIAL NUCLEIC ACID MOLECULES | CureVac AG | 30-
Dec- 13 |
28-
Jun -16 |
20-
Oct- 16 |
| US20160304
882 |
METHOD FOR PROPAGATING ADENOVIRAL VECTORS ENCODING INHIBITORY GENE PRODUCTS | GenVec, Inc. | 10-
Nov- 05 |
27-
Jun -16 |
20-
Oct- 16 |
| US20160304
586 |
PROCESS FOR PREPARING INFLUENZA VACCINES | Crucell Holland B.V. | 5-
Dec- 13 |
4-
Dec -14 |
20-
Oct- 16 |
| US20160304
579 |
POLYPEPTIDES AND USES THEREOF FOR TREATMENT OF AUTOIMMUNE DISORDERS AND INFECTION | Not Available | 30-
Jun- 11 |
29-
Feb -16 |
20-
Oct- 16 |
| US20160304
472 |
Hydrazide Containing Nuclear Transport Modulators and Uses Thereof | Not Available | 29-
Jul-11 |
13-
Nov -15 |
20-
Oct- 16 |
| US20160303
194 |
Compositions And Method For Treatment Of Inflammatory Bowel Disease | Not Available | 2-
Jun- 11 |
21-
Apr -16 |
20-
Oct- 16 |
| US20160303
052 |
MODULAR PARTICLES FOR IMMUNOTHERAPY | Not Available | 1-
Nov- 13 |
31-
Oct -14 |
20-
Oct- 16 |
| US20160299
141 |
NAD ANALOGS AND METHODS OF USING SAID NAD ANALOGS IN DETERMINING RIBOSYLATION OF PROTEINS WITH PARP MUTANTS | Biolog Life Science Institute Forschungslabor und Biochemica-Vertrieb GmbH | 8-
Apr- 15 |
1-
Apr -16 |
13-
Oct- 16 |
| US20160298
179 |
LUMINOPHORE-LABELED MOLECULES COUPLED WITH PARTICLES FOR MICROARRAY-BASED ASSAYS | CapitalBio Corporation | 5-
Dec- 13 |
2-
Dec -14 |
13-
Oct- 16 |
| US20160296
617 |
Immunogenic Composition for MERS Coronavirus Infection | Not Available | 1-
Mar- 13 |
28-
Feb -14 |
13-
Oct- 16 |
| US20160296
616 |
IMMUNOGENIC COMPOSITIONS AND USES THEREOF | Not Available | 25-
Mar- 15 |
25-
Mar -16 |
13-
Oct- 16 |
| US20160295
844 |
GENETICALLY MODIFIED NON-HUMAN ANIMALS AND METHODS OF USE THEREOF | Not Available | 13-
Apr- 15 |
12-
Apr -16 |
13-
Oct- 16 |
| US20160292
393 |
SYSTEMS AND METHODS FOR ORDERING LABORATORY TESTS AND PROVIDING RESULTS THEREOF | Not Available | 24-
Oct- 13 |
13-
Apr -16 |
6-
Oct- 16 |
| US20160289
740 |
METHODS AND COMPOSITIONS FOR COMBINATORIAL BARCODING | Not Available | 30-
Mar- 15 |
29-
Mar -16 |
6-
Oct- 16 |
| US20160289
191 |
ORGANIC COMPOUNDS | NOVARTIS AG | 27-
Jun- 08 |
9-
Oct -15 |
6-
Oct- 16 |
| US20160288
121 |
Slip Chip Device and Methods | Not Available | 24-
Mar- 09 |
25-
May -16 |
6-
Oct- 16 |
| US20160287
697 |
INJECTABLE VACCINE COMPOSITION | NITTO DENKO CORPORATION | 3-
Oct- 13 |
2-
Oct -14 |
6-
Oct- 16 |
| US20160287
622 |
COMPOSITIONS AND METHODS FOR TREATING IMMUNE AND VIRAL DISORDERS AND MODULATING PROTEIN-RNA INTERACTION | Massachusetts Institute of Technology | 18-
Nov- 13 |
7-
Nov -14 |
6-
Oct- 16 |
| US20160281
109 |
GENE TRANSFER INTO AIRWAY EPITHELIAL STEM CELL BY USING LENTIVIRAL VECTOR PSEUDOTYPED WITH RNA VIRUS OR DNA
VIRUS SPIKE PROTEIN |
Not Available | 28-
Oct- 05 |
10-
Jun -16 |
29-
Sep -16 |
| US20160280
707 |
ALKOXY SUBSTITUTED IMIDAZOQUINOLINES | Not Available | 3-
Oct- 03 |
13-
Jun -16 |
29-
Sep -16 |
| US20160279
237 |
ADJUVANT COMPOSITIONS AND RELATED METHODS | Not Available | 24-
Mar- 15 |
24-
Mar -16 |
29-
Sep -16 |
| US20160279
193 |
IMMUNOSUPPRESSIVE AGENTS AND THEIR USE IN THERAPY | Not Available | 6-
Nov- 13 |
6-
Nov -14 |
29-
Sep -16 |
| US20160279
165 |
PULSE INHALATION OF NITRIC OXIDE FOR TREATING RESPIRATORY DISEASES | Not Available | 24-
Mar- 15 |
24-
Mar -16 |
29-
Sep -16 |
| US20160279
163 |
Method of Treating Inflammation | Not Available | 1-
Apr- 10 |
3-
Jun -16 |
29-
Sep -16 |
| US20160278
349 |
Immunocompromised Ungulates | Not Available | 27-
Oct- 08 |
23-
Mar -15 |
29-
Sep -16 |
| US20160272
707 |
VSTM5 ANTIBODIES, AND USES THEREOF FOR TREATMENT OF CANCER, INFECTIOUS DISEASES AND IMMUNE RELATED DISEASES | Not Available | 11-
Sep- 13 |
11-
Sep -14 |
22-
Sep -16 |
| US20160271
241 |
BUNYAVIRUSES WITH SEGMENTED GLYCOPROTEIN PRECURSOR GENES AND METHODS FOR GENERATING THESE VIRUSES | Stichting Dienst Landbouwkundig Onderzoek | 21-
May- 13 |
21-
May -14 |
22-
Sep -16 |
| US20160271
240 |
Tetanus Toxoid and CCL3 Improve DC Vaccines | Duke University | 14-
Nov- 13 |
14-
Nov -15 |
22-
Sep -16 |
| US20160271
137 |
INHIBITORS OF LONG AND VERY LONG CHAIN FATTY ACID METABOLISM AS BROAD SPECTRUM ANTI-VIRALS | Not Available | 18-
Feb- 10 |
27-
Oct -15 |
22-
Sep -16 |
| US20160267
244 |
METHODS OF PREDICTING CANCER LETHALITY USING REPLIKIN COUNTS | Not Available | 8-
Aug- 08 |
19-
May -16 |
15-
Sep -16 |
| US20160265
025 |
NANOREPORTERS AND METHODS OF MANUFACTURING AND USE THEREOF | Not Available | 23-
Dec- 05 |
20-
May -16 |
15-
Sep -16 |
| US20160264
971 |
COMPOSITIONS AND METHODS FOR SILENCING EBOLA VIRUS GENE EXPRESSION | Not Available | 20-
Jul-09 |
9-
Oct -15 |
15-
Sep -16 |
| US20160264
962 |
TAL-EFFECTOR ASSEMBLY PLATFORM, CUSTOMIZED SERVICES, KITS AND ASSAYS | Not Available | 4-
Apr- 12 |
28-
Jul- 15 |
15-
Sep -16 |
| US20160263
156 |
RED BLOOD CELL MEMBRANE-DERIVED MICROPARTICLES AND THEIR USE FOR THE TREATMENT OF LUNG DISEASE | University of Pittsburgh – Of the Com monwealth System of Higher Education | 7-
Nov- 13 |
6-
Nov -14 |
15-
Sep -16 |
| US20160258
949 |
CHIPS, DETECTION SYSTEMS, AND METHODS FOR MULTIPLEX PNEUMOCOCCUS SEROLOGY | Not Available | 9-
Oct- 13 |
9-
Oct -14 |
8-
Sep -16 |
| US20160257
932 |
GENETICALLY ENGINEERED ENUCLEATED ERYTHROID CELLS COMPRISING A PHENYLALANINE AMMONIA LYASE RECEIVER POLYPEPTIDE | Not Available | 18-
Nov- 13 |
17-
May -16 |
8-
Sep -16 |
| US20160257
653 |
Benzazepine Dicarboxamide Compounds | Hoffmann-La Roche Inc. | 6-
Mar- 15 |
4-
Mar -16 |
8-
Sep -16 |
| US20160256
870 |
Slip Chip Device and Methods | Not Available | 24-
Mar- 09 |
25-
May -16 |
8-
Sep -16 |
| US20160256
541 |
Cationic Oil-In-Water Emulsions | Not Available | 6-Jul-
10 |
11-
Mar -16 |
8-
Sep -16 |
| US20160253
584 |
SPATIALLY ADDRESSABLE MOLECULAR BARCODING | Not Available | 27-
Feb- 15 |
26-
Feb -16 |
1-
Sep -16 |
| US20160251
637 |
COMPOSITIONS FOR INCREASING POLYPEPTIDE STABILITY AND ACTIVITY, AND RELATED METHODS | Not Available | 19-
Nov- 09 |
11-
Mar -16 |
1-
Sep -16 |
| US20160251
631 |
DECREASING POTENTIAL IATROGENIC RISKS ASSOCIATED WITH INFLUENZA VACCINES | Novartis AG | 9-
Sep- 04 |
9-
May -16 |
1-
Sep -16 |
| US20160251
399 |
PEPTIDOMIMETIC MACROCYCLES | Not Available | 14-
Jan- 09 |
7-
Apr -16 |
1-
Sep -16 |
| US20160251
362 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.P.A. | 18-
Dec- 12 |
10-
May -16 |
1-
Sep -16 |
| US20160251
319 |
CARBOXYLIC ACID COMPOUNDS | Astrazeneca Aktiebolag | 18-
May- 12 |
11-
May -16 |
1-
Sep -16 |
| US20160250
326 |
Conjugates of GM-CSF and IL-7, and Compositions Thereof | Not Available | 14-
Nov- 11 |
18-
May -16 |
1-
Sep -16 |
| US20160250
278 |
PEPTIDOMIMETIC MACROCYCLES | Not Available | 14-
Jan- 09 |
7-
Apr -16 |
1-
Sep -16 |
| US20160250
168 |
ESTERS OF SHORT CHAINS FATTY ACIDS FOR USE IN THE TREATMENT OF IMMUNOGENIC DISORDERS | Not Available | 3-
Oct- 12 |
3-
Mar -16 |
1-
Sep -16 |
| US20160238
601 |
METHODS AND COMPOSITIONS FOR CORONAVIRUS DIAGNOSTICS AND THERAPEUTICS | Not Available | 14-
Oct- 13 |
14-
Oct -14 |
18-
Aug -16 |
| US20160238
600 |
METHOD FOR SELECTING A SINGLE CELL EXPRESSING A HETEROGENEOUS COMBINATION OF ANTIBODIES | Merus B.V. | 30-
May- 03 |
27-
Apr -16 |
18-
Aug -16 |
| US20160237
455 |
CRISPR-RELATED METHODS AND COMPOSITIONS | Editas Medicine, Inc. | 27-
Sep- 13 |
26-
Sep -14 |
18-
Aug -16 |
| US20160237
123 |
COMPOSITIONS AND METHODS FOR THE TREATMENT OF VIRAL INFECTIONS | Not Available | 23-
Jan- 08 |
4-
May -16 |
18-
Aug -16 |
| US20160237
082 |
DEUBIQUITINASE INHIBITORS AND METHODS FOR USE OF THE SAME | Not Available | 10-
Oct- 13 |
10-
Oct -14 |
18-
Aug -16 |
| US20160235
840 |
Compositions, Comprising Improved Il-12 Genetic Constructs And Vaccines, Immunotherapeutics And Methods Of Using The Same | Not Available | 12-
Dec- 11 |
26-
Feb -16 |
18-
Aug -16 |
| US20160235
837 |
THERAPIES, VACCINES, AND PREDICTIVE METHODS FOR MIDDLE EAST RESPIRATORY SYNDROME VIRUS (MERS CoV) | Not Available | 16-
Oct- 13 |
16-
Oct -14 |
18-
Aug -16 |
| US20160235
835 |
INFLUENZA VACCINES WITH REDUCED AMOUNTS OF SQUALENE | Seqirus UK Limited | 10-
Feb- 09 |
27-
Jan -16 |
18-
Aug -16 |
| US20160235
675 |
Technology for the Preparation of Microparticles | Not Available | 24-
Jul-07 |
24-
Mar -16 |
18-
Aug -16 |
| US20160230
190 |
Lentiviral Vectors Having a Mutated Integrase Protein and uses Thereof | Not Available | 17-
Sep- 13 |
17-
Sep -14 |
11-
Aug -16 |
| US20160229
904 |
Optimized Human Clotting Factor VIII Gene Expression Cassettes and Their Use | Not Available | 6-
Feb- 15 |
5-
Feb -16 |
11-
Aug -16 |
| US20160229
872 |
ISOTHIAZOLOPYRIMIDINONES, PYRAZOLOPYRIMIDINONES, AND PYRROLOPYRIMIDINONES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
4-
Feb -16 |
11-
Aug -16 |
| US20160229
864 |
THIENOPYRIMIDINONES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
4-
Feb -16 |
11-
Aug -16 |
| US20160229
833 |
QUINAZOLINONES AND AZAQUINAZOLINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 5-
Feb- 15 |
4-
Feb -16 |
11-
Aug -16 |
| US20160228
540 |
NASAL MUCOSAL VACCINE COMPOSITION | NITTO DENKO CORPORATION | 3-
Oct- 13 |
2-
Oct -14 |
11-
Aug -16 |
| US20160228
533 |
Use of EGFR Pathway Inhibitors to Increase Immune Responses to Antigens | Emory University | 23-
Sep- 13 |
23-
Sep -14 |
11-
Aug -16 |
| US20160228
532 |
METHOD OF OBTAINING THERMOSTABLE DRIED VACCINE FORMULATIONS | Merck Sharp & Dohme Corp. | 16-
Oct- 13 |
13-
Oct -14 |
11-
Aug -16 |
| US20160228
463 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 16-
Feb- 05 |
20-
Apr -16 |
11-
Aug -16 |
| US20160222
414 |
CONSTRUCTS AND METHODS FOR DELIVERING MOLECULES VIA VIRAL VECTORS WITH BLUNTED INNATE IMMUNE RESPONSES | Not Available | 14-
Mar- 13 |
15-
Apr -16 |
4-
Aug -16 |
| US20160222
072 |
Universal Protein Tag for Double Stranded Nucleic Acid Delivery | Not Available | 23-
Oct- 13 |
22-
Oct -14 |
4-
Aug -16 |
| US20160222
023 |
NOVEL MONOTHIOL MUCOLYTIC AGENTS | PARION SCIENCES, INC. | 30-
Jan- 15 |
29-
Jan -16 |
4-
Aug -16 |
| US20160222
010 |
4-AMINO-IMIDAZOQUINOLINE COMPOUNDS | Hoffmann-La Roche Inc. | 22-
Apr- 14 |
7-
Apr -16 |
4-
Aug -16 |
| US20160221
994 |
Substituted 2,3-Dihydrobenzofuranyl Compounds And Uses Thereof | Not Available | 29-
Nov- 12 |
27-
Nov -13 |
4-
Aug -16 |
| US20160220
664 |
ANTIGEN AND METHOD FOR PRODUCTION THEREOF | Not Available | 13-
Sep- 13 |
12-
Sep -14 |
4-
Aug -16 |
| US20160220
595 |
NUCLEOTIDE AND NUCLEOSIDE THERAPEUTIC COMPOSITIONS AND USES RELATED THERETO | Not Available | 11-
Sep- 13 |
10-
Sep -14 |
4-
Aug -16 |
| US20160220
536 |
USE OF PHENYLMETHIMAZOLES, METHIMAZOLE DERIVATIVES, AND TAUTOMERIC CYCLIC THIONES FOR THE TREATMENT OF AUTOIMMUNE/INFLAMMATORY DISEASES ASSOCIATED WITH TOLLLIKE RECEPTOR OVEREXPRESSION | Not Available | 16-
Mar- 04 |
19-
Feb -16 |
4-
Aug -16 |
| US20160215
282 |
SYNTHETIC ANTISERUM FOR RAPID-TURNAROUND THERAPIES | Not Available | 28-
Jan- 15 |
20-
Jan -16 |
28-
Jul- 16 |
| US20160215
262 |
CD137 ENRICHMENT FOR EFFICIENT TUMOR INFILTRATING LYMPHOCYTE SELECTION | Not Available | 16-
Sep- 13 |
16-
Sep -14 |
28-
Jul- 16 |
| US20160213
776 |
ADSORPTION OF IMMUNOPOTENTIATORS TO INSOLUBLE METAL SALTS | GlaxoSmithKline Biologicals SA | 1-
Sep- 10 |
7-
Apr -16 |
28-
Jul- 16 |
| US20160213
773 |
MUCOSAL VACCINE COMPOSITION | NITTO DENKO CORPORATION | 3-
Oct- 13 |
2-
Oct -14 |
28-
Jul- 16 |
| US20160213
761 |
CARBON NANOTUBE COMPOSITIONS AND METHODS OF USE THEREOF | Not Available | 19-
Mar- 08 |
5-
Apr -16 |
28-
Jul- 16 |
| US20160213
647 |
COMPOSITIONS AND METHODS FOR INHIBITING VIRAL INFECTION | Not Available | 28-
Jan- 15 |
27-
Jan -16 |
28-
Jul- 16 |
| US20160213
610 |
MODIFIED RELEASE FORMULATIONS FOR OPROZOMIB | Not Available | 24-
Oct- 12 |
28-
Jan -16 |
28-
Jul- 16 |
| US20160207
980 |
FC-CONTAINING MOLECULES EXHIBITING PREDICTABLE, CONSISTENT, AND REPRODUCIBLE GLYCOFORM PROFILES | AMGEN INC. | 5-
Sep- 13 |
5-
Sep -14 |
21-
Jul- 16 |
| US20160207
949 |
NOVEL CYTOTOXIC AGENTS FOR CONJUGATION TO A CELL BINDING MOLECULE | Hangzhou DAC Biotech Co., Ltd | 2-
Sep- 13 |
2-
Sep -13 |
21-
Jul- 16 |
| US20160207
900 |
PEPTIDYL NITRIL COMPOUNDS AS DIPEPTIDYL PEPTIDASE I INHIBITORS | PROZYMEX A/S | 9-
Sep- 13 |
8-
Sep -14 |
21-
Jul- 16 |
| US20160206
729 |
IMMUNOGENIC MIDDLE EAST RESPIRATORY SYNDROME CORONAVIRUS (MERS-CoV) COMPOSITIONS AND METHODS | Not Available | 19-
Sep- 13 |
19-
Sep -14 |
21-
Jul- 16 |
| US20160206
719 |
COMBINATION OF VACCINATION AND INHIBITION OF THE PD-1 PATHWAY | CureVac AG | 22-
Feb- 13 |
5-
Apr -16 |
21-
Jul- 16 |
| US20160206
638 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 16-
Feb- 05 |
5-
Apr -16 |
21-
Jul- 16 |
| US20160206
575 |
Inhibition of Biofilm Organisms | NOVABIOTICS LIMITED | 31-
Mar- 09 |
28-
Mar -16 |
21-
Jul- 16 |
| US20160202
258 |
B-CELL ANTIGEN PRESENTING CELL ASSAY | University of Pittsburgh – Of the Com monwealth System of Higher Education | 8-
Apr- 10 |
21-
Mar -16 |
14-
Jul- 16 |
| US20160201
110 |
DIAGNOSIS AND TREATMENT OF INCIPIENT DIABETES | Not Available | 9-
Jan- 15 |
17-
Nov -15 |
14-
Jul- 16 |
| US20160201
088 |
ADENO-ASSOCIATED VIRUS (AAV) SEROTYPE 8 SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 17-
Dec- 01 |
30-
Mar -16 |
14-
Jul- 16 |
| US20160199
486 |
ARRANGING INTERACTION AND BACK PRESSURE CHAMBERS FOR MICROFLUIDIZATION | Not Available | 3-
Dec- 09 |
22-
Mar -16 |
14-
Jul- 16 |
| US20160199
449 |
METHOD OF REDUCING ANTIGENIC DRIFT OR REASSORTMENT OF VIRUSES IN A HOST ANIMAL USING ALPHA INTERFERON | Hemispherx Biopharma, Inc. | 21-
Aug- 13 |
21-
Aug -14 |
14-
Jul- 16 |
| US20160199
416 |
Induced Hepatocytes and Uses Thereof | Not Available | 26-
Nov- 14 |
25-
Nov -15 |
14-
Jul- 16 |
| US20160199
407 |
HALIDES IN THE TREATMENT OF PATHOGENIC INFECTION | THE UNIVERSITY OF IOWA RESEARCH FOUNDATION | 25-
Jan- 08 |
17-
Dec -15 |
14-
Jul- 16 |
| US20160194
387 |
Methods Of Treating Inflammation Associated Airway Diseases And Viral Infections | Not Available | 2-
Jan- 15 |
31-
Dec -15 |
7-
Jul- 16 |
| US20160194
322 |
SUBSTITUTED IMIDAZO RING SYSTEMS AND METHODS | Not Available | 25-
Nov- 03 |
14-
Mar -16 |
7-
Jul- 16 |
| US20160194
278 |
DITHIOL MUCOLYTIC AGENTS | PARION SCIENCES, INC. | 23-
Aug- 13 |
11-
Mar -16 |
7-
Jul- 16 |
| US20160193
603 |
SAMPLE-TO-ANSWER MICROFLUIDIC CARTRIDGE | Not Available | 29-
Jan- 10 |
5-
Aug -15 |
7-
Jul- 16 |
| US20160193
327 |
MUCOSAL VACCINE COMPOSITION | NITTO DENKO CORPORATION | 3-
Oct- 13 |
2-
Oct -14 |
7-
Jul- 16 |
| US20160193
321 |
MAKING INFLUENZA VIRUS VACCINES WITHOUT USING EGGS | Novartis AG | 11-
Sep- 06 |
5-
Nov -15 |
7-
Jul- 16 |
| US20160193
315 |
PEPTIDES SHARED AMONG LETHAL CANCERS AND THERAPEUTIC COMPOSITIONS COMPRISING SAID PEPTIDES | Not Available | 7-
Aug- 09 |
17-
Mar -16 |
7-
Jul- 16 |
| US20160192
658 |
HYDROGEN-CONTAINING ANTIMICROBIAL AGENT | Not Available | 13-
Aug- 13 |
5-
Aug -14 |
7-
Jul- 16 |
| US20160185
786 |
PYRROLOTRIAZINONES AND IMIDAZOTRIAZINONES AS UBIQUITIN- SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 30-
Dec- 14 |
29-
Dec -15 |
30-
Jun- 16 |
| US20160185
785 |
PYRROLO AND PYRAZOLOPYRIMIDINES AS UBIQUITIN-SPECIFIC PROTEASE 7 INHIBITORS | Not Available | 30-
Dec- 14 |
29-
Dec -15 |
30-
Jun- 16 |
| US20160184
424 |
INTRANASAL VACCINATION DOSAGE REGIMEN | Not Available | 17-
Dec- 12 |
17-
Dec -13 |
30-
Jun- 16 |
| US20160184
334 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 16-
Feb- 05 |
11-
Mar -16 |
30-
Jun- 16 |
| US20160177
337 |
METHOD FOR PRODUCTION OF REPROGRAMMED CELL USING CHROMOSOMALLY UNINTEGRATED VIRUS VECTOR | Not Available | 16-
Jul-08 |
8-
Mar -16 |
23-
Jun- 16 |
| US20160177
336 |
Expression Tools for Multiprotein Applications | Not Available | 9-
Mar- 04 |
11-
Dec -15 |
23-
Jun- 16 |
| US20160175
433 |
LIPIDATED IMMUNE RESPONSE MODIFIER COMPOUND COMPOSITIONS, FORMULATIONS, AND METHODS | Not Available | 17-
Aug- 10 |
15-
Dec -15 |
23-
Jun- 16 |
| US20160175
394 |
Compositions and Uses of Lectins | Emory University | 12-
Feb- 10 |
11-
Dec -15 |
23-
Jun- 16 |
| US20160175
387 |
Use of Immune Suppressive Domains as Medicaments | Not Available | 10-
Apr- 13 |
10-
Apr -14 |
23-
Jun- 16 |
| US20160174
631 |
PROTECTIVE MASKS WITH COATING COMPRISING DIFFERENT ELECTROSPUN FIBERS INTERWEAVED WITH EACH OTHER, FORMULATIONS FORMING THE SAME, AND METHOD OF PRODUCING THEREOF | Not Available | 23-
Dec- 14 |
10-
Dec -15 |
23-
Jun- 16 |
| US20160168
203 |
Cyclic Antimicrobial Peptides | NOVABIOTICS LIMITED | 24-
Feb- 06 |
9-
Nov -15 |
16-
Jun- 16 |
| US20160168
101 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.p.A. | 15-
Dec- 14 |
27-
Nov -15 |
16-
Jun- 16 |
| US20160166
710 |
METHOD FOR INCREASING EXPRESSION OF RNA-ENCODED PROTEINS | CureVac AG | 21-
Aug- 13 |
19-
Feb -16 |
16-
Jun- 16 |
| US20160166
676 |
Use of Immune Suppressive Peptides as Adjuvants | Not Available | 10-
Apr- 13 |
10-
Apr -14 |
16-
Jun- 16 |
| US20160160
258 |
MONOCLONAL ANTIBODY PRODUCTION BY EBV TRANSFORMATION OF B CELLS | Not Available | 26-
Feb- 03 |
8-
Feb -16 |
9-
Jun- 16 |
| US20160160
178 |
IMMUNOTHERAPY USING STEM CELLS | Not Available | 9-
Dec- 14 |
2-
Dec -15 |
9-
Jun- 16 |
| US20160159
927 |
IDENTIFICATION OF VSIG8 AS THE PUTATIVE VISTA RECEPTOR (V- R) AND USE THEREOF TO PRODUCE VISTA/VSIG8 AGONISTS AND ANTAGONISTS | Not Available | 5-
Dec- 14 |
7-
Dec -15 |
9-
Jun- 16 |
| US20160158
341 |
PREPARATION OF INFLUENZA VIRUS VACCINE ANTIGENS | Novartis AG | 18-
Mar- 08 |
7-
Dec -15 |
9-
Jun- 16 |
| US20160158
340 |
INFLUENZA VACCINES CONTAINING HEMAGGLUTININ AND MATRIX PROTEINS | Not Available | 27-
Jan- 06 |
4-
Dec -15 |
9-
Jun- 16 |
| US20160158
339 |
METHOD FOR INACTIVATING VIRUSES USING ELECTRON BEAMS | Not Available | 26-
Jul-13 |
24-
Jul- 14 |
9-
Jun- 16 |
| US20160158
308 |
TREATMENT OF MULTIPLE EVOLVING BACTERIAL RESISTANCE DISEASES WITH LIPOSOMALLY FORMULATED GLUTATHIONE | CHILDREN’S HEALTHCARE OF ATLANTA, INC. | 12-
Nov- 13 |
14-
Aug -14 |
9-
Jun- 16 |
| US20160158
294 |
Methods of Populating a Gastrointestinal Tract | Not Available | 4-
Feb- 13 |
4-
Feb -14 |
9-
Jun- 16 |
| US20160158
154 |
PROTEIN VESICLES AND METHODS OF MAKING AND USING THEREOF | Not Available | 5-
Dec- 14 |
7-
Dec -15 |
9-
Jun- 16 |
| US20160153
034 |
Rapid Epidemiologic Typing of Bacteria | Not Available | 19-
May- 08 |
11-
Nov -15 |
2-
Jun- 16 |
| US20160152
693 |
AMINO ACID SEQUENCES DIRECTED AGAINST ENVELOPE PROTEINS OF A VIRUS AND POLYPEPTIDES COMPRISING THE SAME FOR THE TREATMENT OF VIRAL DISEASES | Ablynx N.V. | 5-
Jun- 08 |
29-
Oct -15 |
2-
Jun- 16 |
| US20160152
676 |
HELIX-GRAFTED PROTEINS AS INHIBITORS OF DISEASE-RELEVANT PROTEIN-PROTEIN INTERACTIONS | COLORADO STATE UNIVERSITY RESEARCH FOUNDATION | 7-
Nov- 14 |
9-
Nov -15 |
2-
Jun- 16 |
| US20160152
667 |
COMPOSITIONS AND METHODS FOR THE TREATMENT OF VIRAL INFECTIONS | Not Available | 23-
Jan- 08 |
9-
Feb -16 |
2-
Jun- 16 |
| US20160152
596 |
Nuclear Transport Modulators and Uses Thereof | Karyopharm Therapeutics Inc. | 21-
Jun- 13 |
20-
Jun -14 |
2-
Jun- 16 |
| US20160151
399 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 16-
Feb- 05 |
17-
Feb -16 |
2-
Jun- 16 |
| US20160146
806 |
RECEPTORS FOR B7-H4 | Not Available | 17-
May- 13 |
19-
May -14 |
26-
May -16 |
| US20160146
786 |
Method of monitoring cellular trafficking of peptides | Not Available | 26-
Jun- 13 |
26-
Jun -14 |
26-
May -16 |
| US20160145
246 |
NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 9-
May- 12 |
23-
Jun -15 |
26-
May -16 |
| US20160144
061 |
Compositions and Imaging Methods Comprising Detectably Labeled Phosphatidylethanolamine-Binding Peptides | Not Available | 15-
Jul-02 |
4-
Feb -16 |
26-
May -16 |
| US20160139
143 |
DETECTING TARGETS USING MASS TAGS AND MASS SPECTROMETRY | Not Available | 2-Jul-
10 |
28-
Dec -15 |
19-
May -16 |
| US20160137
702 |
GAS57 MUTANT ANTIGENS AND GAS57 ANTIBODIES | Not Available | 12-
Sep- 07 |
7-
Aug -15 |
19-
May -16 |
| US20160137
649 |
PYRROLO-PYRROLE CARBAMATE AND RELATED ORGANIC COMPOUNDS, PHARMACEUTICAL COMPOSITIONS, AND MEDICAL USES THEREOF | Not Available | 3-Jul-
13 |
1-
Jul- 14 |
19-
May -16 |
| US20160135
453 |
SHELF STABLE, REDUCED CORROSION, READY TO USE PEROXYCARBOXYLIC ACID ANTIMICROBIAL COMPOSITIONS | Not Available | 30-
Aug- 07 |
20-
Jan -16 |
19-
May -16 |
| US20160130
367 |
GENERATION OF BINDING MOLECULES | Merus B.V. | 26-
Sep- 11 |
16-
Sep -15 |
12-
May -16 |
| US20160130
345 |
COMBINATION OF VACCINATION AND INHIBITION OF THE PD-1 PATHWAY | Not Available | 22-
Feb- 13 |
21-
Feb -14 |
12-
May -16 |
| US20160130
265 |
SUBSTITUTED 4-PYRIDONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 23-
Aug- 12 |
29-
Dec -15 |
12-
May -16 |
| US20160129
110 |
IMMUNOPROTECTIVE PRIMARY MESENCHYMAL STEM CELLS AND METHODS | AUTOIMMUNE TECHNOLOGIES, LLC | 14-
Mar- 13 |
17-
Jul- 15 |
12-
May -16 |
| US20160129
104 |
HAND, FOOT, AND MOUTH VACCINES AND METHODS OF MANUFACTURE AND USE THEREOF | Not Available | 7-
Nov- 14 |
6-
Nov -15 |
12-
May -16 |
| US20160129
095 |
Immunostimulatory Combinations | Not Available | 30-
Dec- 02 |
10-
Sep -15 |
12-
May -16 |
| US20160128
937 |
CIRCULATION OF COMPONENTS DURING MICROFLUIDIZATION AND/OR HOMOGENIZATION OF EMULSIONS | Novartis AG | 3-
Dec- 09 |
5-
Sep -14 |
12-
May -16 |
| US20160122
412 |
COMPOSITION COMPRISED OF ANTIGEN LINKED TO A TNF SUPERFAMILY LIGAND | Not Available | 15-
Mar- 13 |
16-
Mar -14 |
5-
May -16 |
| US20160122
397 |
REPLIKIN-BASED COMPOUNDS FOR PREVENTION AND TREATMENT OF INFLUENZA AND METHODS OF DIFFERENTIATING INFECTIVITY AND LETHALITY IN INFLUENZA | Not Available | 23-
Apr- 09 |
2-
Dec -15 |
5-
May -16 |
| US20160122
312 |
ANTI-VIRAL COMPOUNDS, PHARMACEUTICAL COMPOSITIONS AND METHODS OF USE THEREOF | Not Available | 16-
Jul-13 |
16-
Jul- 14 |
5-
May -16 |
| US20160122
306 |
DENDRIMER LIKE AMINO AMIDES POSSESSING SODIUM CHANNEL BLOCKER ACTIVITY FOR THE TREATMENT OF DRY EYE AND OTHER
MUCOSAL DISEASES |
PARION SCIENCES, INC. | 29-
May- 12 |
5-
Jan -16 |
5-
May -16 |
| US20160116
462 |
ANTIBODY-NANOPARTICLE CONJUGATES AND METHODS FOR MAKING AND USING SUCH CONJUGATES | Not Available | 27-
Apr- 10 |
21-
Apr -15 |
28-
Apr- 16 |
| US20160115
522 |
MUTANT PROTEASE BIOSENSORS WITH ENHANCED DETECTION CHARACTERISTICS | Not Available | 11-
May- 10 |
7-
Jan -16 |
28-
Apr- 16 |
| US20160115
221 |
COMPOSITIONS AND METHODS FOR THE TREATMENT OF IMMUNODEFICIENCY | Not Available | 28-
Oct- 14 |
2-
Jul- 15 |
28-
Apr- 16 |
| US20160114
322 |
PARALLELIZED SAMPLE HANDLING | Not Available | 19-
Apr- 13 |
18-
Apr -14 |
28-
Apr- 16 |
| US20160114
037 |
COMPOSITIONS AND METHODS FOR THE TREATMENT OF IMMUNODEFICIENCY | Not Available | 28-
Oct- 14 |
8-
Jan -15 |
28-
Apr- 16 |
| US20160114
027 |
RECOMBINANT HCMV AND RHCMV VECTORS AND USES THEREOF | Not Available | 14-
May- 10 |
1-
Oct -15 |
28-
Apr- 16 |
| US20160114
022 |
THERAPIES, VACCINES, AND PREDICTIVE METHODS FOR FILOVIRUSES INCLUDING EBOLAVIRUS AND MARBURG VIRUS | Not Available | 11-
Oct- 14 |
10-
Oct -15 |
28-
Apr- 16 |
| US20160113
929 |
METHODS AND COMPOSITIONS FOR TREATING VIRAL OR VIRALLY- INDUCED CONDITIONS | Not Available | 11-
Mar- 10 |
2-
Jun -15 |
28-
Apr- 16 |
| US20160113
920 |
COMPOSITIONS AND METHODS FOR INHIBITING BACTERIAL AND VIRAL PATHOGENS | Not Available | 24-
Oct- 14 |
23-
Oct -15 |
28-
Apr- 16 |
| US20160113
881 |
NOVEL NANOPARTICLE COMPOSITIONS | Not Available | 3-
Apr- 13 |
3-
Apr -14 |
28-
Apr- 16 |
| US20160113
870 |
CIRCULATION OF COMPONENTS DURING MICROFLUIDIZATION AND/OR HOMOGENIZATION OF EMULSIONS | Novartis AG | 3-
Dec- 09 |
5-
Sep -14 |
28-
Apr- 16 |
| US20160108
463 |
Biological Specimen Collection and Transport System | Longhorn Vaccines and Diagnostics, LLC | 1-
Oct- 07 |
15-
Dec -15 |
21-
Apr- 16 |
| US20160108
461 |
TARGETED WHOLE GENOME AMPLIFICATION METHOD FOR IDENTIFICATION OF PATHOGENS | Not Available | 14-
Sep- 06 |
5-
Oct -15 |
21-
Apr- 16 |
| US20160108
359 |
AVIAN CELLS FOR IMPROVED VIRUS PRODUCTION | Not Available | 5-
Jun- 13 |
3-
Jun -14 |
21-
Apr- 16 |
| US20160108
097 |
MONOMERIC GRIFFITHSIN TANDEMERS | The United States of America, as represented by the Secretary, Department of Health and Human Serv | 5-
Jun- 13 |
5-
Jun -14 |
21-
Apr- 16 |
| US20160108
096 |
FUSION PROTEINS, RECOMBINANT BACTERIA, AND METHODS FOR USING RECOMBINANT BACTERIA | Not Available | 17-
Sep- 14 |
17-
Sep -15 |
21-
Apr- 16 |
| US20160108
063 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 16-
Feb- 05 |
21-
Dec -15 |
21-
Apr- 16 |
| US20160106
842 |
LIPIDS AND LIPID COMPOSITIONS FOR THE DELIVERY OF ACTIVE AGENTS | Not Available | 8-
Mar- 13 |
6-
Mar -14 |
21-
Apr- 16 |
| US20160102
295 |
MODIFIED ADENOVIRUS HEXON PROTEIN AND USES THEREOF | Not Available | 28-
Apr- 06 |
22-
May -15 |
14-
Apr- 16 |
| US20160102
091 |
HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS | 3-V BIOSCIENCES, INC. | 8-
Mar- 11 |
5-
Oct -15 |
14-
Apr- 16 |
| US20160101
145 |
PEPTIDOMIMETIC MACROCYCLES AND FORMULATIONS THEREOF | Not Available | 24-
Sep- 14 |
24-
Sep -15 |
14-
Apr- 16 |
| US20160096
899 |
METHODS FOR TREATING JUVENILE ARTHRITIS WITH ANTI-BILE SALT-STIMULATED LIPASE (BSSL) ANTIBODIES | Not Available | 8-
Apr- 09 |
28-
Sep -15 |
7-
Apr- 16 |
| US20160096
895 |
BINDING MEMBERS-513 | Not Available | 7-
Nov- 08 |
23-
Oct -15 |
7-
Apr- 16 |
| US20160095
936 |
IMMUNOSTIMULATORY COMPOSITIONS AND METHODS OF USE THEREOF | Not Available | 5-
Apr- 12 |
2-
Jul- 15 |
7-
Apr- 16 |
| US20160090
589 |
COMPOSITIONS FOR AND METHODS OF IDENTIFYING ANTIGENS | Not Available | 21-
Feb- 06 |
6-
May -15 |
31-
Mar -16 |
| US20160090
389 |
Phosphoinositide 3-Kinase Inhibitors | Respivert Ltd. | 15-
Mar- 13 |
4-
Dec -15 |
31-
Mar -16 |
| US20160084
835 |
METHODS FOR DIAGNOSING INFECTIOUS DISEASES USING ADSORPTION MEDIA | EXTHERA MEDICAL CORPORATION | 8-
Nov- 13 |
16-
Oct -15 |
24-
Mar -16 |
| US20160083
748 |
TISSUE PREFERENTIAL CODON MODIFIED EXPRESSION CASSETTES, VECTORS CONTAINING SAME, AND USES THEREOF | Not Available | 29-
Apr- 13 |
29-
Apr -14 |
24-
Mar -16 |
| US20160083
689 |
ANIMAL PROTEIN-FREE MEDIA FOR CULTIVATION OF CELLS | Not Available | 29-
Oct- 04 |
30-
Nov -15 |
24-
Mar -16 |
| US20160082
074 |
COMPOSITIONS AND METHODS FOR TREATING CORONAVIRUS INFECTION | LUDWIG-MAXIMILIANS-UNIVERSITAET MUENCHEN | 11-
Mar- 14 |
8-
May -15 |
24-
Mar -16 |
| US20160081
982 |
METHODS FOR TREATING PULMONARY EMPHYSEMA USING SUBSTITUTED 2-AZA-BICYCLO[2.2.1]HEPTANE-3-CARBOXYLIC ACID (BENZYL-CYANO-METHYL)-AMIDES INHIBITORS OF CATHEPSIN C | Not Available | 14-
Mar- 13 |
1-
Dec -15 |
24-
Mar -16 |
| US20160081
346 |
ANTIMICROBIAL COMPOSITIONS AND METHODS | Not Available | 23-
Sep- 14 |
22-
Sep -15 |
24-
Mar -16 |
| US20160077
094 |
Media Elaborated with Newly Synthesized Antibodies (MENSA) and Uses Thereof | Not Available | 5-
May- 14 |
5-
May -15 |
17-
Mar -16 |
| US20160076
094 |
Efficient Deep Sequencing and Rapid Genomic Speciation of RNA Viruses (vRNAseq) | Not Available | 8-
Sep- 14 |
1-
Sep -15 |
17-
Mar -16 |
| US20160076
053 |
REPLICATION DEFECTIVE ADENOVIRUS VECTOR IN VACCINATION | Not Available | 24-
Aug- 12 |
15-
Mar -13 |
17-
Mar -16 |
| US20160075
704 |
NOVEL SUBSTITUTED SPIROCYCLES | Not Available | 12-
Sep- 14 |
10-
Sep -15 |
17-
Mar -16 |
| US20160074
507 |
METHOD FOR PREPARING VIRAL PARTICLES WITH CYCLIC DINUCLEOTIDE AND USE OF SAID PARTICLES FOR INDUCING IMMUNE RESPONSE | Not Available | 16-
Sep- 14 |
16-
Sep -15 |
17-
Mar -16 |
| US20160074
506 |
DELIVERY OF SELF-REPLICATING RNA USING BIODEGRADABLE POLYMER PARTICLES | Not Available | 6-Jul-
10 |
23-
Nov -15 |
17-
Mar -16 |
| US20160074
481 |
Clottable Concentrate Of Platelet Growth Factors And Preparation Method Thereof | Zheng Yang Biomedical Technology Co., LTD. | 7-
Jan- 08 |
30-
Nov -15 |
17-
Mar -16 |
| US20160068
843 |
Compositions and Methods for „Resistance-Proof” SiRNA Therapeutics for Influenza | Sirnaomics, Inc. | 8-Jul-
12 |
7-
Jul- 13 |
10-
Mar -16 |
| US20160068
587 |
OLIGOPEPTIDE-FREE CELL CULTURE MEDIA | Not Available | 3-
Jan- 07 |
16-
Nov -15 |
10-
Mar -16 |
| US20160068
573 |
PEPTIDOMIMETIC MACROCYCLES | Not Available | 14-
Jan- 09 |
14-
Sep -15 |
10-
Mar -16 |
| US20160067
333 |
GENERATING PEPTOID VACCINES | The Board of Regents of the University of Texas System | 3-
May- 13 |
2-
May -14 |
10-
Mar -16 |
| US20160060
598 |
MEANS AND METHODS FOR INFLUENCING THE STABILITY OF ANTIBODY PRODUCING CELLS | Not Available | 9-
Dec- 05 |
26-
Aug -15 |
3-
Mar -16 |
| US20160060
231 |
SUBSTITUTED BICYCLIC DIHYDROPYRIMIDINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 6-
Feb- 13 |
9-
Nov -15 |
3-
Mar -16 |
| US20160060
230 |
SUBSTITUTED BICYCLIC DIHYDROPYRIMIDINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 6-
Feb- 13 |
9-
Nov -15 |
3-
Mar -16 |
| US20160060
224 |
N-MYRISTOYL TRANSFERASE INHIBITORS | UNIVERSITY OF DUNDEE | 2-
Sep- 08 |
31-
Aug -15 |
3-
Mar -16 |
| US20160058
814 |
PLANT EXTRACTS AND RELATED COMPOSITIONS, METHODS AND SYSTEMS | Not Available | 26-
Aug- 14 |
26-
Aug -14 |
3-
Mar -16 |
| US20160058
012 |
WEAR RESISTANT ANTIMICROBIAL COMPOSITIONS AND METHODS OF USE | Not Available | 14-
Dec- 10 |
24-
Sep -15 |
3-
Mar -16 |
| US20160058
008 |
ANTIMICROBIAL COMPOSITIONS AND METHODS WITH NOVEL POLYMERIC BINDING SYSTEM | Not Available | 28-
Aug- 14 |
28-
Aug -15 |
3-
Mar -16 |
| US20160054
312 |
CHEMICALLY DIFFERENTIATED SENSOR ARRAY | Nanomedical Diagnostics, Inc. | 28-
Apr- 14 |
15-
Oct -15 |
25-
Feb -16 |
| US20160053
308 |
PROBE KIT FOR DETECTING A SINGLE STRAND TARGET NUCLEOTIDE SEQUENCE | Fondzione Istituto Italiano Di Tecnolgia | 27-
Dec- 12 |
27-
Dec -13 |
25-
Feb -16 |
| US20160053
222 |
BACILLUS BASED DELIVERY SYSTEM AND METHODS OF USE | Not Available | 18-
Apr- 11 |
9-
Sep -15 |
25-
Feb -16 |
| US20160052
959 |
RADIOLABELED CATIONIC STEROID ANTIMICROBIALS AND DIAGNOSTIC METHODS | BRIGHAM YOUNG UNIVERSITY | 22-
Aug- 14 |
19-
Aug -15 |
25-
Feb -16 |
| US20160051
691 |
CARBOHYDRATE CONJUGATES AS DELIVERY AGENTS FOR OLIGONUCLEOTIDES | Not Available | 4-
Dec- 07 |
25-
Aug -15 |
25-
Feb -16 |
| US20160051
670 |
METHODS FOR PREPARING SQUALENE | Not Available | 12-
May- 10 |
23-
Oct -15 |
25-
Feb -16 |
| US20160051
669 |
COMPOSITIONS AND METHODS FOR SELECTIVELY MODULATING TREGS | Not Available | 21-
Aug- 14 |
21-
Aug -15 |
25-
Feb -16 |
| US20160046
705 |
HETERODIMERIC IMMUNOGLOBULINS | Not Available | 21-
Nov- 12 |
21-
Nov -13 |
18-
Feb -16 |
| US20160046
687 |
GM-CSF AND IL-4 CONJUGATES, COMPOSITIONS, AND METHODS RELATED THERETO | Not Available | 23-
Oct- 12 |
23-
Oct -13 |
18-
Feb -16 |
| US20160045
547 |
METHOD OF PREVENTING OR TREATING SINUSITIS WITH OXIDATIVE REDUCTIVE POTENTIAL WATER SOLUTION | OCULUS INNOVATIVE SCIENCES, INC. | 30-
Dec- 03 |
27-
Oct -15 |
18-
Feb -16 |
| US20160041
168 |
ARRAYED DETECTOR SYSTEM FOR MEASUREMENT OF INFLUENZA IMMUNE RESPONSE | Not Available | 2-
May- 08 |
23-
Oct -15 |
11-
Feb -16 |
| US20160040
161 |
In Vivo Delivery of Oligonucleotides | OncoImmunin Inc. | 12-
Dec- 11 |
12-
Dec -12 |
11-
Feb -16 |
| US20160039
867 |
SINGLE-CHAIN ANTIPARALLEL COILED COIL PROTEINS | Complix NV | 8-
Dec- 08 |
21-
Aug -15 |
11-
Feb -16 |
| US20160039
860 |
ENANTIOMERS OF THE 1′,6′-ISOMER OF NEPLANOCIN A | Not Available | 4-
Aug- 14 |
4-
Aug -15 |
11-
Feb -16 |
| US20160039
826 |
2-((4-AMINO-3-(3-FLUORO-5-HYDROXYPHENYL)-1H- PYRAZOLO[3,4-D]PYRIMIDIN-1 -YL)METHYL)-3-(2- (TRIFLUOROMETHYL)BENZYL) QUINAZOLIN-4(3H)-ONE DERIVATIVES AND THEIR USE AS PHOSPHOINOSITIDE 3-KINASE INHIBITORS | Not Available | 15-
Mar- 13 |
14-
Mar -14 |
11-
Feb -16 |
| US20160039
812 |
SUBSTITUTED BICYCLIC DIHYDROPYRIMIDINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 31-
Jul-14 |
27-
Jul- 15 |
11-
Feb -16 |
| US20160032
319 |
VECTORS COMPRISING STUFFER/FILLER POLYNUCLEOTIDE SEQUENCES AND METHODS OF USE | THE CHILDREN’S HOSPITAL OF PHILADELPHIA | 15-
Mar- 13 |
14-
Mar -14 |
4-
Feb -16 |
| US20160031
861 |
SUBSTITUTED OXETANES AND THEIR USE AS INHIBITORS OF CATHEPSIN C | Not Available | 1-
Aug- 14 |
31-
Jul- 15 |
4-
Feb -16 |
| US20160031
831 |
SUBSTITUTED BICYCLIC DIHYDROPYRIMIDINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 31-
Jul-14 |
27-
Jul- 15 |
4-
Feb -16 |
| US20160031
830 |
SUBSTITUTED BICYCLIC DIHYDROPYRIMIDINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 31-
Jul-14 |
27-
Jul- 15 |
4-
Feb -16 |
| US20160031
829 |
SUBSTITUTED BICYCLIC DIHYDROPYRIMIDINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 31-
Jul-14 |
27-
Jul- 15 |
4-
Feb -16 |
| US20160031
825 |
SUBSTITUTED DIHYDROPYRIMIDINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 31-
Jul-14 |
27-
Jul- 15 |
4-
Feb -16 |
| US20160030
443 |
METHODS AND COMPOSITIONS FOR MODULATING REGULATORY T CELL FUNCTION | Not Available | 14-
Mar- 13 |
14-
Mar -14 |
4-
Feb -16 |
| US20160025
675 |
METHOD FOR ELECTRONIC BIOLOGICAL SAMPLE ANALYSIS | Nanomedical Diagnostics, Inc. | 28-
Apr- 14 |
1-
Oct -15 |
28-
Jan- 16 |
| US20160025
603 |
AIRBORNE AGENT COLLECTORS, METHODS, SYSTEMS AND DEVICES FOR MONITORING AIRBORNE AGENTS | Not Available | 22-
Jul-14 |
21-
Jul- 15 |
28-
Jan- 16 |
| US20160024
525 |
REVERSE GENETICS USING NON-ENDOGENOUS POL I PROMOTERS | NOVARTIS AG | 21-
May- 09 |
7-
May -15 |
28-
Jan- 16 |
| US20160024
158 |
Inhibitory Polypeptides Specific to WNT Inhibitors | Not Available | 15-
Mar- 13 |
14-
Mar -14 |
28-
Jan- 16 |
| US20160022
801 |
RODENT HEPADNAVIRUS CORES WITH REDUCED CARRIER- SPECIFIC ANTIGENICITY | Not Available | 15-
Mar- 13 |
14-
Mar -14 |
28-
Jan- 16 |
| US20160016
999 |
INHIBITORY PEPTIDES OF VIRAL INFECTION | Not Available | 17-
Jul-14 |
16-
Jul- 15 |
21-
Jan- 16 |
| US20160016
986 |
STABILIZED NUCLEOTIDES FOR MEDICAL TREATMENT | Not Available | 21-
Jul-14 |
21-
Jul- 15 |
21-
Jan- 16 |
| US20160016
916 |
Exo Olefin-Containing Nuclear Transport Modulators and Uses Thereof | Not Available | 15-
Mar- 13 |
14-
Mar -14 |
21-
Jan- 16 |
| US20160015
826 |
Constructs Binding to Phosphatidylserine and Their Use in Disease Treatment and Imaging | Not Available | 24-
Jan- 05 |
2-
Feb -15 |
21-
Jan- 16 |
| US20160015
803 |
IMMUNOSTIMULATORY COMBINATIONS AND USE THEREOF | Not Available | 18-
Jul-14 |
17-
Jul- 15 |
21-
Jan- 16 |
| US20160011
183 |
MULTIANALYTE ASSAY | Not Available | 30-
Apr- 07 |
15-
Jun -15 |
14-
Jan- 16 |
| US20160008
809 |
METHODS AND COMPOSITIONS FOR PAPER-BASED AND HYBRID MICROFLUIDIC DEVICES INTEGRATED WITH NUCLEIC ACID AMPLIFICATION FOR DISEASE DIAGNOSIS | University of Texas at El Paso | 10-
Jul-14 |
10-
Jul- 15 |
14-
Jan- 16 |
| US20160008
461 |
METHODS OF GENERATING ROBUST PASSIVE AND ACTIVE IMMUNE RESPONSES | Not Available | 8-
Jan- 13 |
2-
Aug -14 |
14-
Jan- 16 |
| US20160008
451 |
NANOPARTICLE-BASED COMPOSITIONS | Not Available | 14-
Mar- 13 |
14-
Mar -14 |
14-
Jan- 16 |
| US20160008
397 |
IMMUNOMODULATION BY CONTROLLING EXPRESSION LEVELS OF MICRORNAS IN DENDRITIC CELLS | Not Available | 14-
Jul-14 |
10-
Jul- 15 |
14-
Jan- 16 |
| US20160008
363 |
DRUG COMBINATION | VERONA PHARMA PLC | 15-
Mar- 13 |
17-
Mar -14 |
14-
Jan- 16 |
| US20160003
747 |
Apparatus for two-step surface-enhanced raman spectroscopy | REAL-TIME ANALYZERS, INC | 16-
Dec- 11 |
11-
Sep -15 |
7-
Jan- 16 |
| US20160002
608 |
METHODS TO PRODUCE BUNYAVIRUS REPLICON PARTICLES | Not Available | 20-
Sep- 10 |
10-
Jul- 15 |
7-
Jan- 16 |
| US20160000
905 |
Nanoparticle Delivery of TLR Agonists and Antigens | Not Available | 25-
Feb- 13 |
24-
Feb -14 |
7-
Jan- 16 |
| US20160000
790 |
DRUG COMBINATION | VERONA PHARMA PLC | 15-
Mar- 13 |
17-
Mar -14 |
7-
Jan- 16 |
| US20160000
754 |
Antiviral Activity from Medicinal Mushrooms and their Active Constituents | Not Available | 6-
Jan- 04 |
14-
Sep -15 |
7-
Jan- 16 |
| US20150377
887 |
IN SITU AFFINITY MATURATION OF ANTIBODIES | Not Available | 27-
Feb- 13 |
27-
Feb -14 |
31-
Dec -15 |
| US20150376
621 |
MODIFIED SMALL INTERFERING RNA MOLECULES AND METHODS OF USE | ARROWHEAD RESEARCH CORPORATION | 28-
Feb- 08 |
14-
May -15 |
31-
Dec -15 |
| US20150376
584 |
METHODS OF TREATING VIRAL INFECTIONS, PARTICULARLY RABIES, MERS-COV, INFLUENZA, EBOLA, CHIKUNGUNYA, VENEZUELAN EQUINE ENCEPHALITUS, CANINE PARVOVIRUS, ADENOVIRUS, RESPIRATORY SYNCYTIAL VIRUS, RHINOVIRUS, AND POXVIRUS IN MAMMALIAN PATIENTS | TAMIR BIOTECHNOLOGY, INC. | 28-
Mar- 14 |
10-
Jun -15 |
31-
Dec -15 |
| US20150376
145 |
STABLE SODIUM CHANNEL BLOCKERS | PARION SCIENCES, INC. | 30-
Jun- 14 |
30-
Jun -15 |
31-
Dec -15 |
| US20150376
131 |
SUBSTITUTED 4-PYRIDONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 23-
Aug- 12 |
2-
Sep -15 |
31-
Dec -15 |
| US20150374
723 |
Anti-Viral Azide Containing Compounds | Not Available | 28-
Jul-10 |
20-
Aug -15 |
31-
Dec -15 |
| US20150374
626 |
TREATMENT OF EVOLVING BACTERIAL RESISTANCE DISEASES INCLUDING KLEBSIELLA PNEUMONIAE WITH LIPOSOMALLY FORMULATED GLUTATHIONE | YOUR ENERGY SYSTEMS, LLC | 15-
Feb- 13 |
15-
Mar -13 |
31-
Dec -15 |
| US20150369
806 |
DETECTION OF VIRAL DISEASES USING A BIOCHIP THAT CONTAINS GOLD NANOPARTICLES | Not Available | 19-
Jun- 14 |
17-
Jun -15 |
24-
Dec -15 |
| US20150368
661 |
METHODS FOR PRODUCING ANTIBODIES | Not Available | 11-
Feb- 13 |
11-
Feb -14 |
24-
Dec -15 |
| US20150368
294 |
GADD45BETA TARGETING AGENTS | Not Available | 22-
Oct- 09 |
10-
Feb -15 |
24-
Dec -15 |
| US20150366
888 |
SUBSTITUTED NUCLEOSIDES, NUCLEOTIDES AND ANALOGS THEREOF | Not Available | 24-
Jun- 14 |
22-
Jun -15 |
24-
Dec -15 |
| US20150366
796 |
INTRADERMAL DELIVERY OF IMMUNOLOGICAL COMPOSITIONS COMPRISING TOLL-LIKE RECEPTOR AGONISTS | Not Available | 1-
Feb- 13 |
30-
Jan -14 |
24-
Dec -15 |
| US20150361
432 |
MODIFIED SMALL INTERFERING RNA MOLECULES AND METHODS OF USE | Not Available | 26-
Jul-02 |
6-
Aug -15 |
17-
Dec -15 |
| US20150361
097 |
HETEROCYCLIC AMIDE DERIVATIVES AS P2X7 RECEPTOR ANTAGONISTS | Not Available | 22-
Jan- 13 |
21-
Jan -14 |
17-
Dec -15 |
| US20150361
096 |
HETEROCYCLIC AMIDE DERIVATIVES AS P2X7 RECEPTOR ANTAGONISTS | Not Available | 22-
Jan- 13 |
21-
Jan -14 |
17-
Dec -15 |
| US20150359
871 |
IMMUNOGENIC COMPOSITIONS COMPRISING SILICIFIED VIRUS AND METHODS OF USE | PORTLAND STATE UNIVERSITY | 31-
Jan- 13 |
31-
Jan -14 |
17-
Dec -15 |
| US20150359
746 |
Technology for the Preparation of Microparticles | Not Available | 24-
Jul-07 |
17-
Jun -15 |
17-
Dec -15 |
| US20150355
172 |
ADP-RIBOSE DETECTION REAGENTS | The Board of Regents of the University of Texas System | 10-
Jun- 14 |
9-
Jun -15 |
10-
Dec -15 |
| US20150353
905 |
CAS9-NUCLEIC ACID COMPLEXES AND USES RELATED THERETO | Not Available | 16-
Jan- 13 |
15-
Jan -14 |
10-
Dec -15 |
| US20150353
596 |
17-Substituted Steroid Compounds | Not Available | 28-
Aug- 02 |
24-
Aug -15 |
10-
Dec -15 |
| US20150353
561 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.P.A. | 9-
Jun- 14 |
5-
Jun -15 |
10-
Dec -15 |
| US20150352
218 |
HYDRAZINO 1H-IMIDAZOQUINOLIN-4-AMINES AND CONJUGATES MADE THEREFROM | 3M INNOVATIVE PROPERTIES COMPANY | 3-
Jun- 11 |
14-
Aug -15 |
10-
Dec -15 |
| US20150344
425 |
INDOLE CARBOXAMIDE DERIVATIVES AS P2X7 RECEPTOR ANTAGONISTS | ACTELION PHARMACEUTICALS LTD | 18-
Dec- 12 |
17-
Dec -13 |
3-
Dec -15 |
| US20150343
028 |
METHODS OF MODULATING IMMUNE RESPONSES BY MODIFYING AKT3 BIOACTIVITY | Not Available | 17-
Apr- 14 |
17-
Apr -15 |
3-
Dec -15 |
| US20150337
369 |
SINGLE CELL ANALYSIS OF T CELLS USING HIGH-THROUGHPUT MULTIPLEX AMPLIFICATION AND DEEP SEQUENCING | Not Available | 7-
May- 14 |
30-
Apr -15 |
26-
Nov -15 |
| US20150337
334 |
METHOD FOR PRODUCTION OF REPROGRAMMED CELL USING CHROMOSOMALLY UNINTEGRATED VIRUS VECTOR | Not Available | 16-
Jul-08 |
29-
Jul- 15 |
26-
Nov -15 |
| US20150337
015 |
ANTIVIRAL RIFT VALLEY FEVER VIRUS PEPTIDES AND METHODS OF
USE |
The United States of America, as represented by the Secretary of the Army, on behalf of the United | 6-
Dec- 12 |
4-
Jun -15 |
26-
Nov -15 |
| US20150335
733 |
Immunogenic Composition and Methods of Using the Compositions for Inducing Humoral and Cellular Immune Responses | Not Available | 8-
Apr- 11 |
27-
Apr -15 |
26-
Nov -15 |
| US20150335
728 |
METHODS AND COMPOSITIONS FOR IMMUNIZATION AGAINST VIRUS | Academia Sinica | 27-
Mar- 09 |
18-
Feb -14 |
26-
Nov -15 |
| US20150335
657 |
Methods for Modulating Sirtuin Enzymes | Not Available | 5-
May- 14 |
4-
May -15 |
26-
Nov -15 |
| US20150329
866 |
NOVEL siRNAS AND METHODS OF USE THEREOF | Not Available | 25-
Oct- 06 |
8-
Jan -15 |
19-
Nov -15 |
| US20150329
834 |
METHODS OF PROPAGATING MONKEY ADENOVIRAL VECTORS | GenVec, Inc. | 9-
Nov- 09 |
4-
Aug -15 |
19-
Nov -15 |
| US20150328
282 |
METHODS FOR TREATING VIRAL DISORDERS | Not Available | 24-
Sep- 09 |
5-
Aug -15 |
19-
Nov -15 |
| US20150322
491 |
High density self-contained biological analysis | Not Available | 15-
Nov- 06 |
16-
Jul- 15 |
12-
Nov -15 |
| US20150322
155 |
ACETYLENEDICARBOXYL LINKERS AND THEIR USES IN SPECIFIC CONJUGATION OF A CELL-BINDING MOLECULE | Robert Yongxin Zhao | 15-
Jul-15 |
15-
Jul- 15 |
12-
Nov -15 |
| US20150322
137 |
HUMAN ANTIBODY SPECIFIC TO HUMAN METAPNEUMOVIRUS, OR ANTIGEN-BINDING FRAGMENT THEREOF | Not Available | 28-
Jan- 13 |
28-
Jan -14 |
12-
Nov -15 |
| US20150322
022 |
THERAPEUTIC CATECHOLS | RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY | 12-
May- 14 |
11-
May -15 |
12-
Nov -15 |
| US20150322
008 |
INDOLE CARBOXAMIDE DERIVATIVES AS P2X7 RECEPTOR ANTAGONIST | ACTELION PHARMACEUTICALS LTD | 12-
Dec- 12 |
11-
Dec -13 |
12-
Nov -15 |
| US20150320
842 |
A COMPOSITION FOR PREVENTING OR TREATING AN RNA VIRAL INFECTION COMPRISING SAMHD1 OR A NUCLEIC ACID MOLECULE ENCODING THE SAMHD1 | SNU R&DB FOUNDATION | 7-
Jan- 13 |
7-
Jan -13 |
12-
Nov -15 |
| US20150320
801 |
USE OF ASC AND ASC-CM TO TREAT ARDS, SARS, AND MERS | Not Available | 6-
May- 14 |
6-
May -15 |
12-
Nov -15 |
| US20150315
612 |
ADENO-ASSOCIATED VIRUS (AAV) CLADES, SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 30-
Sep- 03 |
20-
Jul- 15 |
5-
Nov -15 |
| US20150315
574 |
PRODUCTION OF STABLE NON-POLYADENYLATED RNAS | Massachusetts Institute of Technology | 16-
Oct- 12 |
16-
Oct -13 |
5-
Nov -15 |
| US20150314
017 |
DISULFUR BRIDGE LINKERS FOR CONJUGATION OF A CELLBINDING MOLECULE | Dr. Robert Yongxin Zhao | 15-
Jul-15 |
15-
Jul- 15 |
5-
Nov -15 |
| US20150313
960 |
NOVEL DEPSIPEPTIDE AND USES THEREOF | Not Available | 3-
Dec- 12 |
1-
Jul- 15 |
5-
Nov -15 |
| US20150313
929 |
USE OF TYLVALOSIN AS ANTIVIRAL AGENT | CAMBRIDGE UNIVERSITY TECHNICAL SERVICES | 13-
Jul-06 |
8-
Jun -15 |
5-
Nov -15 |
| US20150313
909 |
ANTIVIRAL COMPOUNDS AND METHODS | Biotron Limited | 26-
Jun- 03 |
6-
Feb -15 |
5-
Nov -15 |
| US20150309
018 |
SYSTEM AND METHOD FOR ELECTRONIC BIOLOGICAL SAMPLE ANALYSIS | NANOMEDICAL DIAGNOSTICS, INC. | 28-
Apr- 14 |
28-
Apr -14 |
29-
Oct- 15 |
| US20150307
936 |
SYSTEM AND METHOD FOR DNA SEQUENCING AND BLOOD CHEMISTRY ANALYSIS | Nanomedical Diagnostics, Inc. | 28-
Apr- 14 |
10-
Apr -15 |
29-
Oct- 15 |
| US20150307
935 |
NON-MASS DETERMINED BASE COMPOSITIONS FOR NUCLEIC ACID DETECTION | Not Available | 6-
Aug- 09 |
13-
Jul- 15 |
29-
Oct- 15 |
| US20150307
849 |
HIGHLY EFFICIENT INFLUENZA MATRIX (M1) PROTEINS | Not Available | 11-
Jul-03 |
23-
Feb -15 |
29-
Oct- 15 |
| US20150307
572 |
BIOACTIVE PEPTIDES AND METHODS OF USING SAME | Not Available | 12-
Jul-07 |
23-
Feb -15 |
29-
Oct- 15 |
| US20150307
530 |
NOVEL MUCOLYTIC AGENTS | PARION SCIENCES, INC. | 31-
Aug- 12 |
30-
Aug -13 |
29-
Oct- 15 |
| US20150306
213 |
HMGB1-DERIVED PEPTIDES ENHANCE IMMUNE RESPONSE TO ANTIGENS | The Regents of the University of California | 27-
Jul-10 |
9-
Mar -15 |
29-
Oct- 15 |
| US20150306
212 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
12-
Jun -15 |
29-
Oct- 15 |
| US20150306
205 |
VACCINE COMPOSITION FOR NAIVE SUBJECTS | Not Available | 17-
Dec- 12 |
17-
Dec -13 |
29-
Oct- 15 |
| US20150306
137 |
METHODS OF TREATING OR PREVENTING INFLAMMATION AND HYPERSENSITIVITY WITH OXIDATIVE REDUCTIVE POTENTIAL
WATER SOLUTION |
OCULUS INNOVATIVE SCIENCES, INC. | 20-
Jan- 06 |
7-
Jul- 15 |
29-
Oct- 15 |
| US20150301
055 |
CIRCULATING BIOMARKERS FOR DISEASE | Not Available | 6-
Apr- 10 |
1-
Jul- 15 |
22-
Oct- 15 |
| US20150299
728 |
TC-83-DERIVED ALPHAVIRUS VECTORS, PARTICLES AND METHODS | Not Available | 18-
May- 04 |
6-
Jul- 15 |
22-
Oct- 15 |
| US20150299
707 |
CONSTRUCTION OF POOL OF INTERFERING NUCLEIC ACIDS COVERING ENTIRE RNA TARGET SEQUENCE AND RELATED COMPOSITIONS | BIOMICS BIOTECHNOLOGIES CO., LTD. | 7-Jul-
08 |
2-
Jul- 15 |
22-
Oct- 15 |
| US20150299
667 |
INFLUENZA VIRUS AND TYPE 1 DIABETES | ISTITUTO ZOOPROFILATTICO SPERIMENTALE DELLE VENEZIE | 10-
Oct- 12 |
10-
Oct -13 |
22-
Oct- 15 |
| US20150299
271 |
TREATING CANCER WITH VIRAL NUCLEIC ACID | Not Available | 20-
Feb- 07 |
6-
Jul- 15 |
22-
Oct- 15 |
| US20150299
194 |
4-AMINO-IMIDAZOQUINOLINE COMPOUNDS | HOFFMANN-LA ROCHE INC. | 22-
Apr- 14 |
22-
Apr -15 |
22-
Oct- 15 |
| US20150299
142 |
3,5-DIAMINO-6-CHLORO-N-(4-PHENYLBUTYL)CARBAMIMIDOYL) PYRAZINE-2-CARBOXAMIDE COMPOUNDS | PARION SCIENCES, INC. | 17-
Dec- 12 |
8-
Jan -15 |
22-
Oct- 15 |
| US20150297
700 |
Novel Adenovirus Vectors | Not Available | 10-
Apr- 07 |
27-
Apr -15 |
22-
Oct- 15 |
| US20150297
677 |
COMPOSITIONS AND METHODS FOR INHIBITING VIRAL ENTRY | Children Medical Center Corporation | 13-
Dec- 12 |
12-
Dec -13 |
22-
Oct- 15 |
| US20150297
668 |
ANALOGS OF C5a AND METHODS OF USING SAME | BOARD PF REGENTS OF THE UNIVERSITY OF NEBRASKA | 29-
Jun- 10 |
29-
Jun -11 |
22-
Oct- 15 |
| US20150291
609 |
MERTK-SPECIFIC PYRIMIDINE COMPOUNDS | Not Available | 11-
Apr- 14 |
3-
Apr -15 |
15-
Oct- 15 |
| US20150291
606 |
MERTK-SPECIFIC PYRROLOPYRIMIDINE COMPOUNDS | Not Available | 11-
Apr- 14 |
3-
Apr -15 |
15-
Oct- 15 |
| US20150291
605 |
MERTK-SPECIFIC PYRAZOLOPYRIMIDINE COMPOUNDS | Not Available | 11-
Apr- 14 |
3-
Apr -15 |
15-
Oct- 15 |
| US20150291
531 |
THERAPEUTIC HYDROXYQUINOLONES | RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY | 9-
Nov- 12 |
8-
Nov -13 |
15-
Oct- 15 |
| US20150290
235 |
USE OF SMALL MOLECULE INHIBITORS/ACTIVATORS IN COMBINATION WITH (DEOXY)NUCLEOSIDE OR (DEOXY)NUCLEOTIDE ANALOGS FOR TREATMENT OF CANCER AND HEMATOLOGICAL MALIGNANCIES OR VIRAL INFECTIONS | Not Available | 23-
Nov- 12 |
8-
Nov -13 |
15-
Oct- 15 |
| US20150290
234 |
TARGETED INTRACELLULAR DELIVERY OF ANTIVIRAL AGENTS | Not Available | 23-
Mar- 07 |
21-
Apr -15 |
15-
Oct- 15 |
| US20150290
212 |
THERAPEUTIC USES OF SELECTED PYRAZOLOPYRIMIDINE COMPOUNDS WITH ANTI-MER TYROSINE KINASE ACTIVITY | Not Available | 11-
Apr- 14 |
3-
Apr -15 |
15-
Oct- 15 |
| US20150290
197 |
THERAPEUTIC USES OF SELECTED PYRROLOPYRIMIDINE COMPOUNDS WITH ANTI-MER TYROSINE KINASE ACTIVITY | Not Available | 11-
Apr- 14 |
3-
Apr -15 |
15-
Oct- 15 |
| US20150290
194 |
THERAPEUTIC USES OF SELECTED PYRIMIDINE COMPOUNDS WITH ANTI-MER TYROSINE KINASE ACTIVITY | Not Available | 11-
Apr- 14 |
3-
Apr -15 |
15-
Oct- 15 |
| US20150290
189 |
CHEMICALLY AND METABOLICALLY STABLE DIPEPTIDE POSSESSING POTENT SODIUM CHANNEL BLOCKER ACTIVITY | PARION SCIENCES, INC. | 27-
Jun- 11 |
12-
Jan -15 |
15-
Oct- 15 |
| US20150290
134 |
TECHNOLOGY FOR PREPARATION OF MACROMOLECULAR MICROSPHERES | Not Available | 24-
Jan- 06 |
21-
Apr -15 |
15-
Oct- 15 |
| US20150289
573 |
SELF SANITIZING FACE MASKS AND METHOD OF MANUFACTURE | Not Available | 1-
Jun- 07 |
26-
Jun -15 |
15-
Oct- 15 |
| US20150284
475 |
BISPECIFIC ANTIBODY | Not Available | 21-
Nov- 12 |
21-
Nov -12 |
8-
Oct- 15 |
| US20150284
451 |
COMPOSITIONS AND METHODS FOR INHIBITING PATHOGEN INFECTION | Not Available | 29-
Oct- 12 |
29-
Oct -13 |
8-
Oct- 15 |
| US20150284
416 |
NOVEL LINKERS FOR CONJUGATION OF CELL-BINDING MOLECULES | SUZHOU M-CONJ BIOTECH CO., LTD | 16-
Jun- 15 |
16-
Jun -15 |
8-
Oct- 15 |
| US20150283
531 |
Microspotting Device | Not Available | 18-
Apr- 12 |
17-
Apr -13 |
8-
Oct- 15 |
| US20150283
233 |
Compositions and Methods for Enhancing Immune Responses | Not Available | 15-
Jun- 12 |
17-
Jun -13 |
8-
Oct- 15 |
| US20150283
221 |
ATTENUATED LISTERIA MONOCYTOGENES MUTANT AS A VACCINE VECTOR FOR THE DELIVERY OF EXOGENEOUS ANTIGENS | The Board of Trustees of the University of Illinois | 7-
Apr- 14 |
7-
Apr -15 |
8-
Oct- 15 |
| US20150275
183 |
Human Betacoronavirus Lineage C and Identification of N-Terminal Dipeptidyl Peptidase As Its Virus Receptor | Not Available | 23-
Sep- 12 |
23-
Sep -13 |
1-
Oct- 15 |
| US20150274
698 |
Hydrazide Containing Nuclear Transport Modulators And Uses Thereof | Not Available | 29-
Jul-11 |
10-
Jun -15 |
1-
Oct- 15 |
| US20150272
988 |
INHALATION OF NITRIC OXIDE FOR TREATING RESPIRATORY DISEASES | Advanced Inhalation Therapies (AIT) Ltd. | 7-
Mar- 12 |
7-
Mar -13 |
1-
Oct- 15 |
| US20150267
245 |
PRESERVATION OF BIOLOGICAL MATERIALS IN NON-AQUEOUS FLUID MEDIA | GenTegra, LLC | 14-
Mar- 13 |
14-
Mar -14 |
24-
Sep -15 |
| US20150267
202 |
ANTISENSE ANTIVIRAL COMPOUND AND METHOD FOR TREATING ss/RNA VIRAL INFECTION | Not Available | 16-
Sep- 04 |
6-
Nov -14 |
24-
Sep -15 |
| US20150267
194 |
DOUBLE-STRANDED OLIGONUCLEOTIDE MOLECULES TO DDIT4 AND METHODS OF USE THEREOF | Quark Pharmaceutical, Inc. | 12-
Sep- 12 |
12-
Sep -13 |
24-
Sep -15 |
| US20150266
929 |
VIRUS-LIKE PARTICLES, METHODS OF PREPARATION, AND IMMUNOGENIC COMPOSITIONS | Not Available | 17-
May- 02 |
4-
May -15 |
24-
Sep -15 |
| US20150266
901 |
ALKYLOXY SUBSTITUTED THIAZOLOQUINOLINES AND THIAZOLONAPHTHYRIDINES | Not Available | 9-
Feb- 05 |
8-
Jun -15 |
24-
Sep -15 |
| US20150266
882 |
PYRAZOLO[4,3-D]PYRIMIDINES AS KINASE INHIBITORS | Not Available | 19-
Oct- 12 |
18-
Oct -13 |
24-
Sep -15 |
| US20150265
721 |
Compounds and Methods for Modulating an Immune Response | Not Available | 23-
Mar- 09 |
23-
Dec -14 |
24-
Sep -15 |
| US20150265
697 |
HIGH-YIELD TRANSGENIC MAMMALIAN EXPRESSION SYSTEM FOR GENERATING VIRUS-LIKE PARTICLES | Academia Sinica | 5-
Sep- 06 |
11-
Feb -15 |
24-
Sep -15 |
| US20150265
696 |
Compositions And Methods For Treating And Preventing Porcine Reproductive And Respiratory Syndrome | Not Available | 24-
Apr- 12 |
23-
Feb -15 |
24-
Sep -15 |
| US20150259
427 |
GITR BINDING MOLECULES AND USES THEREFOR | Not Available | 25-
Mar- 05 |
23-
Mar -15 |
17-
Sep -15 |
| US20150259
399 |
ANTIBODIES AND PROCESSES FOR PREPARING THE SAME | Not Available | 16-
May- 08 |
18-
Mar -15 |
17-
Sep -15 |
| US20150259
340 |
NOVEL KINASE INHIBITORS | Not Available | 19-
Oct- 12 |
18-
Oct -13 |
17-
Sep -15 |
| US20150259
292 |
HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS | Not Available | 8-
Mar- 11 |
19-
Mar -15 |
17-
Sep -15 |
| US20150258
191 |
ARRANGING INTERACTION AND BACK PRESSURE CHAMBERS FOR MICROFLUIDIZATION | Novartis AG | 3-
Dec- 09 |
13-
Mar -15 |
17-
Sep -15 |
| US20150252
439 |
Methods and Compositions for Prostate Cancer Metastasis | Not Available | 25-
Mar- 11 |
20-
May -15 |
10-
Sep -15 |
| US20150252
080 |
FUSION PROTEINS FOR PROMOTING AN IMMUNE RESPONSE, NUCLEIC ACIDS ENCODING SAME, AND METHODS OF MAKING AND USE THEREOF | Not Available | 7-
Sep- 12 |
9-
Sep -13 |
10-
Sep -15 |
| US20150250
896 |
HYDROPHILIC LINKERS AND THEIR USES FOR CONJUGATION OF DRUGS TO A CELL BINDING MOLECULES | Hangzhou DAC Biotech Co., Ltd. | 24-
Nov- 12 |
24-
Nov -12 |
10-
Sep -15 |
| US20150247
190 |
METHODS AND SYSTEMS FOR MICROFLUIDICS IMAGING AND ANALYSIS | Not Available | 5-
Oct- 12 |
4-
Oct -13 |
3-
Sep -15 |
| US20150246
110 |
ADJUVANTED INFLUENZA VACCINES INCLUDING CYTOKINE- INDUCING AGENTS | Novartis AG | 4-
Nov- 05 |
14-
May -15 |
3-
Sep -15 |
| US20150246
091 |
COMPOUNDS AND METHODS FOR PREVENTING OR TREATING A VIRAL INFECTION | Not Available | 2-
Nov- 07 |
15-
May -15 |
3-
Sep -15 |
| US20150246
015 |
Esters of Short Chains Fatty Acids for Use in the Treatment of Immunogenic Disorders | Not Available | 3-
Oct- 12 |
3-
Oct -13 |
3-
Sep -15 |
| US20150239
940 |
COMPOSITIONS AND METHODS FOR VIRUS INHIBITION | AUTOIMMUNE TECHNOLOGIES, LLC | 4-
Nov- 03 |
13-
May -15 |
27-
Aug -15 |
| US20150239
875 |
SUBSTITUTED PYRIDONES AND PYRAZINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | Not Available | 21-
Feb- 14 |
12-
Feb -15 |
27-
Aug -15 |
| US20150238
550 |
AAV Vectors Targeted to Oligodendrocytes | Not Available | 28-
Sep- 12 |
27-
Sep -13 |
27-
Aug -15 |
| US20150238
489 |
HETEROCYCLYL CARBOXAMIDES FOR TREATING VIRAL DISEASES | Not Available | 31-
Aug- 12 |
30-
Aug -13 |
27-
Aug -15 |
| US20150232
957 |
MODIFIED OLIGONUCLEOTIDES COMPRISING THIOL FUNCTIONS AND USE THEREOF FOR DETECTING NUCLEIC ACIDS | Not Available | 4-
Apr- 12 |
4-
Apr -13 |
20-
Aug -15 |
| US20150232
881 |
CRISPR-RELATED METHODS AND COMPOSITIONS WITH GOVERNING gRNAS | EDITAS MEDICINE, INC. | 7-
Nov- 13 |
7-
Nov -14 |
20-
Aug -15 |
| US20150232
878 |
Construct | Not Available | 28-
Mar- 06 |
20-
Oct -14 |
20-
Aug -15 |
| US20150232
454 |
THERAPEUTIC HYDROXYPYRIDINONES, HYDROXYPYRIMIDINONES AND HYDROXYPYRIDAZINONES | RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY | 11-
Sep- 12 |
11-
Sep -13 |
20-
Aug -15 |
| US20150231
232 |
OUTER MEMBRANE VESICLES | Not Available | 18-
Sep- 12 |
18-
Sep -13 |
20-
Aug -15 |
| US20150225
432 |
COMPOUNDS AND COMPOSITIONS AS TLR ACTIVITY MODULATORS | Novartis AG | 2-
Sep- 09 |
24-
Apr -15 |
13-
Aug -15 |
| US20150218
162 |
SUBSTITUTED IMIDAZOQUINOLINES, IMIDAZOPYRIDINES, AND IMIDAZONAPHTHYRIDINES | Not Available | 18-
Jun- 04 |
13-
Apr -15 |
6-
Aug -15 |
| US20150218
109 |
C-REL INHIBITORS AND USES THEREOF | Not Available | 21-
Sep- 12 |
19-
Sep -13 |
6-
Aug -15 |
| US20150216
973 |
METHOD FOR THE INDUCTION OF AN IMMUNE RESPONSE | Not Available | 8-
Aug- 12 |
8-
Aug -13 |
6-
Aug -15 |
| US20150211
006 |
CHIRAL CONTROL | Not Available | 13-
Jul-12 |
12-
Jul- 13 |
30-
Jul- 15 |
| US20150210
733 |
NUCLEIC ACID CHEMICAL MODIFICATIONS | Not Available | 2-
Mar- 09 |
22-
Jan -15 |
30-
Jul- 15 |
| US20150210
655 |
CERTAIN (2S)-N-[(1S)-1-CYANO-2-PHENYLETHYL]-1,4- OXAZEPANE-2-CARBOXAMIDES AS DIPEPTIDYL PEPTIDASE 1 INHIBITORS | ASTRAZENECA AB | 24-
Jan- 14 |
21-
Jan -15 |
30-
Jul- 15 |
| US20150203
847 |
CHEMICAL MODIFICATIONS OF MONOMERS AND OLIGONUCLEOTIDES WITH CYCLOADDITION | Not Available | 23-
Sep- 08 |
31-
Dec -14 |
23-
Jul- 15 |
| US20150203
816 |
MEANS AND METHODS FOR INFLUENCING THE STABILITY OF CELLS | Not Available | 4-
Dec- 09 |
26-
Mar -15 |
23-
Jul- 15 |
| US20150202
284 |
METHOD OF MAKING A VACCINE | The United States of America, as represented by the Secretary, Dept. of Health & Human Services | 11-
Oct- 08 |
20-
Jan -15 |
23-
Jul- 15 |
| US20150196
619 |
EV576 FOR USE IN THE TREATMENT OF VIRAL INFECTIONS OF THE RESPIRATORY TRACT | Not Available | 8-
Jan- 10 |
2-
Jan -15 |
16-
Jul- 15 |
| US20150196
578 |
COMBINATION THERAPY TREATMENT FOR VIRAL INFECTIONS | Not Available | 14-
Oct- 09 |
10-
Dec -14 |
16-
Jul- 15 |
| US20150196
032 |
ANTIVIRAL COMPOSITIONS | Long Island University | 30-
May- 07 |
19-
Mar -15 |
16-
Jul- 15 |
| US20150191
704 |
POXVIRUS-PLASMODIUM RECOMBINANTS, COMPOSITIONS CONTAINING SUCH RECOMBINANTS, USES THEREOF, AND METHODS OF MAKING AND USING SAME | Not Available | 30-
Dec- 13 |
19-
Dec -14 |
9-
Jul- 15 |
| US20150191
607 |
Anti-fouling Paints and Coatings | REACTIVE SURFACES, LTD | 3-Jul-
03 |
4-
Dec -13 |
9-
Jul- 15 |
| US20150184
231 |
BIOAGENT DETECTION OLIGONUCLEOTIDES | Not Available | 27-
Dec- 11 |
27-
Dec -12 |
2-
Jul- 15 |
| US20150183
837 |
IMMUNOGENIC COMPOSITIONS AND METHODS OF USE THEREOF | Not Available | 17-
Dec- 07 |
13-
Feb -15 |
2-
Jul- 15 |
| US20150182
636 |
GENETICALLY MODIFIED HUMAN UMBILICAL CORD PERIVASCULAR CELLS FOR PROPHYLAXIS AGAINST OR TREATMENT OF BIOLOGICAL OR CHEMICAL AGENTS | Not Available | 21-
Apr- 08 |
13-
Mar -15 |
2-
Jul- 15 |
| US20150182
588 |
SYNTHETIC MEMBRANE-RECEIVER COMPLEXES | Not Available | 18-
Nov- 13 |
23-
Dec -14 |
2-
Jul- 15 |
| US20150176
027 |
ADENO-ASSOCIATED VIRUS (AAV) SEROTYPE 8 SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | Not Available | 17-
Dec- 01 |
16-
Jan -15 |
25-
Jun- 15 |
| US20150175
656 |
CRYSTALLINE TRIPEPTIDE EPOXY KETONE PROTEASE INHIBITORS | Not Available | 20-
Mar- 09 |
5-
Mar -15 |
25-
Jun- 15 |
| US20150174
257 |
METHODS AND REAGENTS FOR EFFICIENT AND TARGETED GENE TRANSFER TO MONOCYTES AND MACROPHAGES | Not Available | 29-
Apr- 09 |
3-
Mar -15 |
25-
Jun- 15 |
| US20150174
206 |
USES OF INTERFERONS WITH ALTERED SPATIAL STRUCTURE | Not Available | 28-
Aug- 03 |
28-
Jan -15 |
25-
Jun- 15 |
| US20150174
198 |
GENE TRANSFER INTO AIRWAY EPITHELIAL STEM CELL BY USING LENTIVIRAL VECTOR PSEUDOTYPED WITH RNA VIRUS OR DNA
VIRUS SPIKE PROTEIN |
Not Available | 28-
Oct- 05 |
17-
Nov -14 |
25-
Jun- 15 |
| US20150174
158 |
INHALATION OF NITRIC OXIDE FOR TREATING RESPIRATORY DISEASES | Not Available | 7-
Mar- 12 |
7-
Mar -13 |
25-
Jun- 15 |
| US20150173
366 |
PHYSICAL ANTIMICROBIAL METHOD | NMS TECHNOLOGIES CO., LTD. | 1-
Aug- 12 |
16-
Jul- 13 |
25-
Jun- 15 |
| US20150168
405 |
OLIGONUCLEOTIDE BASED ANALYTE DETECTION METHOD | Not Available | 16-
Feb- 10 |
24-
Feb -15 |
18-
Jun- 15 |
| US20150166
989 |
PARTICLE-NUCLEIC ACID CONJUGATES AND THERAPEUTIC USES RELATED THERETO | Not Available | 25-
Jun- 12 |
27-
Feb -13 |
18-
Jun- 15 |
| US20150166
966 |
REVERSE GENETICS METHODS FOR VIRUS RESCUE | Not Available | 20-
Oct- 09 |
1-
Oct -14 |
18-
Jun- 15 |
| US20150166
548 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.p.A. | 16-
Dec- 13 |
16-
Dec -14 |
18-
Jun- 15 |
| US20150166
532 |
HOST TARGETED INHIBITORS OF DENGUE VIRUS AND OTHER VIRUSES | Not Available | 11-
Apr- 12 |
15-
Mar -13 |
18-
Jun- 15 |
| US20150166
488 |
ARYLALKYL- AND ARYLOXYALKYL-SUBSTITUTED EPTHELIAL SODIUM CHANNEL BLOCKING COMPOUNDS | PARION SCIENCES, INC. | 13-
Dec- 13 |
19-
Dec -14 |
18-
Jun- 15 |
| US20150166
487 |
ARYLALKYL- AND ARYLOXYALKYL-SUBSTITUTED EPITHELIAL SODIUM CHANNEL BLOCKING COMPOUNDS | Parion Sciences, Inc. | 13-
Dec- 13 |
13-
Dec -13 |
18-
Jun- 15 |
| US20150165
019 |
Saccharide Conjugate Vaccines | Not Available | 24-
Dec- 04 |
5-
Aug -14 |
18-
Jun- 15 |
| US20150165
009 |
TLR5 LIGANDS, THERAPEUTIC METHODS, AND COMPOSITIONS RELATED THERETO | Emory University | 24-
Sep- 10 |
20-
Sep -11 |
18-
Jun- 15 |
| US20150164
800 |
REGULATING THE INTERACTION BETWEEN TAM LIGANDS AND LIPID MEMBRANES WITH EXPOSED PHOSPHATIDYL SERINE | Salk Institute For Biological Studies | 25-
Jul-12 |
23-
Jul- 13 |
18-
Jun- 15 |
| US20150159
205 |
METHODS AND REAGENTS FOR AMPLIFYING NUCLEIC ACIDS | The United States of America, as represented by the Secretary, Department of Health and Human | 6-
Aug- 12 |
6-
Aug -12 |
11-
Jun- 15 |
| US20150159
173 |
METHOD OF INCREASING THE FUNCTION OF AN AAV VECTOR | The Trustees of the University of Pennsylvania | 7-
Apr- 05 |
18-
Feb -15 |
11-
Jun- 15 |
| US20150157
636 |
METHODS FOR TREATING VIRAL DISORDERS | Not Available | 24-
Sep- 09 |
18-
Feb -15 |
11-
Jun- 15 |
| US20150152
149 |
Peptides Having Activity of Inhibiting Infections of Respiratory Viruses and Use of the Same | Not Available | 9-
May- 13 |
21-
Feb -14 |
4-
Jun- 15 |
| US20150150
963 |
VACCINATION WITH INTERLEUKIN-4 ANTAGONISTS | THE AUSTRALIAN NATIONAL UNIVERSITY | 5-
Jun- 12 |
5-
Jun -13 |
4-
Jun- 15 |
| US20150150
893 |
ANDROGRAPHOLIDE ANALOGS AND THEIR USE FOR MEDICATION | Not Available | 18-
Jun- 12 |
31-
May -13 |
4-
Jun- 15 |
| US20150150
878 |
Methods For Inhibiting Viruses By Targeting Cathepsin-L Cleavage Sites In The Viruses’ Glycoproteins | Not Available | 4-
Apr- 12 |
4-
Apr -13 |
4-
Jun- 15 |
| US20150148
402 |
MODIFIED SMALL INTERFERING RNA MOLECULES AND METHODS OF USE | NOVARTIS AG | 1-
Oct- 04 |
9-
May -14 |
28-
May -15 |
| US20150147
346 |
REPLIKIN SEQUENCES AND THEIR ANTIBODIES FOR DIAGNOSTICS, THERAPEUTICS, AND VACCINES AGAINST PRION AND NEURODEGENERATIVE DISORDERS INCLUDING ALZHEIMER’S DISEASE | Not Available | 2-
May- 12 |
1-
May -13 |
28-
May -15 |
| US20150141
625 |
IMMUNE RESPONSE MODIFIER CONJUGATES | Not Available | 22-
Feb- 06 |
20-
Jan -15 |
21-
May -15 |
| US20150141
387 |
PHARMACEUTICAL PRODUCT COMPRISING A P38 KINASE INHIBITOR AND A SECOND ACTIVE INGREDIENT | Not Available | 18-
Dec- 08 |
14-
Jan -15 |
21-
May -15 |
| US20150140
121 |
Compositions and Methods for Tight Junction Modulation | Not Available | 8-
Jun- 12 |
10-
Jun -13 |
21-
May -15 |
| US20150139
949 |
ANTI-VIRAL COMBINATION THERAPY | Not Available | 6-
Apr- 11 |
19-
May -14 |
21-
May -15 |
| US20150133
633 |
MODIFICATION OF PEPTIDES USING A BIS(THIOETHER)ARYLBRIDGE APPROACH | Not Available | 18-
May- 12 |
17-
May -13 |
14-
May -15 |
| US20150133
402 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 20-
Jun- 07 |
7-
Nov -14 |
14-
May -15 |
| US20150133
391 |
CELL-FREE NUCLEIC ACIDS FOR THE ANALYSIS OF THE HUMAN MICROBIOME AND COMPONENTS THEREOF | Not Available | 7-
Nov- 13 |
7-
Nov -14 |
14-
May -15 |
| US20150132
339 |
ADJUVANTED FORMULATIONS OF STREPTOCOCCUS PNEUMONIAE ANTIGENS | Not Available | 7-
Mar- 12 |
7-
Mar -13 |
14-
May -15 |
| US20150132
337 |
COMBINATION GAS VACCINES AND THERAPEUTICS | Not Available | 17-
Sep- 08 |
13-
Nov -14 |
14-
May -15 |
| US20150132
220 |
Compositions and Imaging Methods Comprising Detectably Labeled Phosphatidylethanolamine-Binding Peptides | Not Available | 15-
Jul-02 |
8-
Jan -15 |
14-
May -15 |
| US20150126
722 |
OLIGONUCLEOTIDE COMPOUND AND METHOD FOR TREATING NIDOVIRUS INFECTIONS | Not Available | 24-
Dec- 03 |
4-
Jun -14 |
7-
May -15 |
| US20150125
540 |
RELEASE OF AGENTS FROM CELLS | Not Available | 15-
Oct- 09 |
5-
Jan -15 |
7-
May -15 |
| US20150125
502 |
DISINFECTING COMPOSITION AND WIPES WITH REDUCED CONTACT TIME | Not Available | 6-
Nov- 13 |
4-
Nov -14 |
7-
May -15 |
| US20150125
483 |
FUSION PROTEINS OF CILIATE GRANULE LATTICE PROTEINS, GRANULAR PROTEIN PARTICLES THEREOF, AND USES THEREFOR | Tetragenetics, Inc. | 27-
May- 11 |
29-
May -12 |
7-
May -15 |
| US20150125
475 |
IMMUNOLOGICALLY USEFUL ARGININE SALTS | Not Available | 7-
Mar- 12 |
7-
Mar -13 |
7-
May -15 |
| US20150125
384 |
MODULAR NANODEVICES FOR SMART ADAPTABLE VACCINES | Not Available | 15-
Feb- 07 |
10-
Nov -14 |
7-
May -15 |
| US20150119
445 |
Carbohydrate Conjugates as Delivery Agents for Oligonucleotides | Not Available | 4-
Dec- 07 |
22-
Jul- 14 |
30-
Apr- 15 |
| US20150119
444 |
Carbohydrate Conjugates as Delivery Agents for Oligonucleotides | Not Available | 4-
Dec- 07 |
11-
Jul- 14 |
30-
Apr- 15 |
| US20150119
426 |
MODULATORS OF THE RELAXIN RECEPTOR 1 | Not Available | 4-
May- 12 |
15-
Mar -13 |
30-
Apr- 15 |
| US20150119
364 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 16-
Feb- 05 |
10-
Nov -14 |
30-
Apr- 15 |
| US20150119
318 |
PEPTIDE COMPOSITIONS AND METHODS FOR INHIBITING HERPESVIRUS INFECTION | THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND | 30-
Oct- 09 |
9-
Jul- 14 |
30-
Apr- 15 |
| US20150118
746 |
INFLUENZA VIRUS REASSORTMENT METHOD | Not Available | 21-
May- 10 |
24-
Oct -14 |
30-
Apr- 15 |
| US20150118
319 |
PROTEOLYSIS-RESISTANT CAPSID OF CHIMERIC HEPATITIS E VIRUS AS AN ORAL DELIVERY VECTOR | Not Available | 27-
Feb- 09 |
5-
Nov -14 |
30-
Apr- 15 |
| US20150118
265 |
MODIFIED ERYTHROCYTE PRECURSOR CELLS AND USES THEREOF | Anthrogenesis Corporation | 13-
Mar- 12 |
12-
Mar -13 |
30-
Apr- 15 |
| US20150118
264 |
PHARMACEUTICAL COMPOSITION COMPRISING A POLYMERIC CARRIER CARGO COMPLEX AND AT LEAST ONE PROTEIN OR PEPTIDE ANTIGEN | CureVac GMBH | 31-
Jan- 12 |
31-
Jan -13 |
30-
Apr- 15 |
| US20150118
222 |
TREATMENT USING BRUTON’S TYROSINE KINASE INHIBITORS AND IMMUNOTHERAPY | Not Available | 25-
Oct- 13 |
24-
Oct -14 |
30-
Apr- 15 |
| US20150118
201 |
Directed Evolution and In Vitro Panning of Virus Vectors | Not Available | 30-
Apr- 08 |
22-
Oct -14 |
30-
Apr- 15 |
| US20150118
183 |
NEGATIVELY CHARGED NUCLEIC ACID COMPRISING COMPLEXES FOR IMMUNOSTIMULATION | CUREVAC GMBH | 31-
Jan- 12 |
31-
Jan -13 |
30-
Apr- 15 |
| US20150111
955 |
AAV VECTOR COMPOSITIONS AND METHODS FOR GENE TRANSFER TO CELLS, ORGANS AND TISSUES | Not Available | 17-
Feb- 12 |
19-
Feb -13 |
23-
Apr- 15 |
| US20150111
945 |
COMPOSITIONS AND METHODS FOR SILENCING EBOLA VIRUS GENE EXPRESSION | Not Available | 20-
Jul-09 |
28-
Mar -14 |
23-
Apr- 15 |
| US20150111
893 |
Nuclear Transport Modulators and Uses Thereof | Not Available | 9-
May- 12 |
9-
May -13 |
23-
Apr- 15 |
| US20150110
807 |
HDC-SIGN BINDING PEPTIDES | Sloan-Kettering Institute for Cancer Research | 19-
Dec- 11 |
10-
Dec -12 |
23-
Apr- 15 |
| US20150105
375 |
METHODS FOR TREATING PULMONARY EMPHYSEMA USING SUBSTITUTED 2-AZA-BICYCLO[2.2.1]HEPTANE-3-CARBOXYLIC ACID (BENZYL-CYANO-METHYL)-AMIDES INHIBITORS OF CATHEPSIN C | Not Available | 14-
Mar- 13 |
18-
Dec -14 |
16-
Apr- 15 |
| US20150104
867 |
ANIMAL PROTEIN-FREE MEDIA FOR CULTIVATION OF CELLS | Baxter Healthcare SA | 29-
Oct- 04 |
2-
May -14 |
16-
Apr- 15 |
| US20150104
500 |
Methods and Compositions for Preventing a Condition | Not Available | 9-
Aug- 10 |
28-
Aug -14 |
16-
Apr- 15 |
| US20150099
770 |
CARBOXYLIC ACID COMPOUNDS | Not Available | 18-
May- 12 |
17-
May -13 |
9-
Apr- 15 |
| US20150099
769 |
PYRROLO[3,2-D]PYRIMIDIN-4-ONE DERIVATIVES AND THEIR USE IN THERAPY | Not Available | 6-
Dec- 04 |
15-
Sep -14 |
9-
Apr- 15 |
| US20150099
764 |
DENDRIMER LIKE AMINO AMIDES POSSESSING SODIUM CHANNEL BLOCKER ACTIVITY FOR THE TREATMENT OF DRY EYE AND OTHER
MUCOSAL DISEASES |
PARION SCIENCES, INC. | 29-
May- 12 |
9-
Dec -14 |
9-
Apr- 15 |
| US20150099
656 |
Multiplex Immuno Screening Assay | Not Available | 4-
May- 12 |
3-
May -13 |
9-
Apr- 15 |
| US20150099
263 |
SELECTIVE DETECTION OF HUMAN RHINOVIRUS | Not Available | 5-
Dec- 08 |
15-
Dec -14 |
9-
Apr- 15 |
| US20150099
261 |
RESPIRATORY INFECTION ASSAY | Not Available | 9-
Dec- 11 |
10-
Dec -12 |
9-
Apr- 15 |
| US20150093
413 |
NUCLEIC ACID COMPRISING OR CODING FOR A HISTONE STEM- LOOP AND A POLY(A) SEQUENCE OR A POLYADENYLATION SIGNAL FOR INCREASING THE EXPRESSION OF AN ENCODED PATHOGENIC ANTIGEN | CureVac GmbH | 15-
Feb- 12 |
15-
Feb -13 |
2-
Apr- 15 |
| US20150086
576 |
SORTASE-MODIFIED VHH DOMAINS AND USES THEREOF | Not Available | 13-
Apr- 12 |
15-
Apr -13 |
26-
Mar -15 |
| US20150080
447 |
Sceletium Extract and Uses Thereof | H. L. Hall & Sons Limited | 20-
Mar- 09 |
15-
Jul- 14 |
19-
Mar -15 |
| US20150080
396 |
Novel Pyrimidine Derivatives and Their Use in the Treatment of Cancer and Further Diseases | ASTRAZENECA AB | 21-
May- 09 |
26-
Aug -14 |
19-
Mar -15 |
| US20150080
344 |
PHOSPHONATES WITH REDUCED TOXICITY FOR TREATMENT OF VIRAL INFECTIONS | Not Available | 14-
Apr- 10 |
29-
Sep -14 |
19-
Mar -15 |
| US20150080
319 |
Inhibition Of Tace Activity With Cyclic Peptides | Not Available | 2-
Jun- 11 |
19-
Sep -14 |
19-
Mar -15 |
| US20150079
155 |
Cationic Liposomal Drug Delivery System for Specific Targeting of Human CD14+ Monocytes in Whole Blood | Not Available | 14-
Mar- 12 |
14-
Mar -13 |
19-
Mar -15 |
| US20150079
121 |
Human Respiratory Syncytial Virus Consensus Antigens, Nucleic Acid Constructs And Vaccines Made Therefrom, And Methods Of Using Same | Not Available | 10-
Apr- 12 |
10-
Apr -13 |
19-
Mar -15 |
| US20150073
136 |
PYRAZINONE DERIVATIVES | Not Available | 27-
Jun- 07 |
17-
Nov -14 |
12-
Mar -15 |
| US20150072
973 |
NOVEL SELECTIVE INHIBITORS OF UBIQUITIN SPECIFIC PROTEASE 7, THE PHARMACEUTICAL COMPOSITIONS THEREOF AND THEIR THERAPEUTIC APPLICATIONS | Not Available | 13-
Aug- 10 |
17-
Sep -14 |
12-
Mar -15 |
| US20150072
023 |
INHALATION OF NITRIC OXIDE FOR TREATING RESPIRATORY DISEASES | Not Available | 11-
Sep- 13 |
11-
Sep -14 |
12-
Mar -15 |
| US20150065
459 |
BORON-CONTAINING SMALL MOLECULES | Not Available | 16-
Feb- 05 |
10-
Nov -14 |
5-
Mar -15 |
| US20150065
458 |
Composition for Inactivating an Enveloped Virus | Not Available | 19-
May- 06 |
17-
Nov -14 |
5-
Mar -15 |
| US20150064
137 |
USE OF ENGINEERED VIRUSES TO SPECIFICALLY KILL SENESCENT CELLS | Kythera Biopharmaceuticals, Inc. | 17-
Apr- 12 |
16-
Apr -13 |
5-
Mar -15 |
| US20150056
636 |
Transgenic Immunodeficient Mouse Expressing Human SIRP-alpha | Not Available | 26-
Mar- 12 |
26-
Mar -13 |
26-
Feb -15 |
| US20150056
305 |
DITHIOL MUCOLYTIC AGENTS | PARION SCIENCES, INC. | 23-
Aug- 13 |
13-
Aug -14 |
26-
Feb -15 |
| US20150051
206 |
COMPOUNDS AND COMPOSITIONS AS C-KIT KINASE INHIBITORS | IRM LLC | 1-
Sep- 11 |
29-
Aug -12 |
19-
Feb -15 |
| US20150050
713 |
Technology for the Preparation of Microparticles | Not Available | 24-
Jul-07 |
25-
Jul- 14 |
19-
Feb -15 |
| US20150050
308 |
CORONAVIRUS, NUCLEIC ACID, PROTEIN, AND METHODS FOR THE GENERATION OF VACCINE, MEDICAMENTS AND DIAGNOSTICS | Not Available | 18-
Aug- 03 |
13-
Aug -14 |
19-
Feb -15 |
| US20150050
278 |
SOLUBLE ENGINEERED MONOMERIC FC | Not Available | 16-
Mar- 12 |
14-
Mar -13 |
19-
Feb -15 |
| US20150045
412 |
USE OF THE CHROMOSOME 19 MICRORNA CLUSTER (C19MC) FOR TREATING MICROBIAL DISEASE AND PROMOTING AUTHOPHAGY | Not Available | 7-
Mar- 12 |
6-
Mar -13 |
12-
Feb -15 |
| US20150044
768 |
METHODS AND COMPOSITIONS FOR PRODUCTION OF RECOMBINANT PROTEIN IN HBX-EXPRESSING MAMMALIAN CELLS | Not Available | 28-
May- 08 |
24-
Oct -14 |
12-
Feb -15 |
| US20150044
305 |
INHALATION OF NITRIC OXIDE FOR TREATING RESPIRATORY DISEASES | Not Available | 7-
Mar- 12 |
7-
Mar -13 |
12-
Feb -15 |
| US20150044
279 |
METHODS AND COMPOSITIONS FOR ENHANCING IMMUNE RESPONSE | Not Available | 10-
Apr- 03 |
6-
Oct -14 |
12-
Feb -15 |
| US20150037
288 |
METHODS FOR INCREASING THE INFECTIVITY OF VIRUSES | Not Available | 26-
Jan- 12 |
25-
Jan -13 |
5-
Feb -15 |
| US20150037
281 |
VARIANTS OF PROTHYMOSIN ALPHA AND METHODS OF USING SAME | Icahn School of Medicine at Mount Sinai | 2-
Mar- 12 |
1-
Mar -13 |
5-
Feb -15 |
| US20150034
084 |
INHALATION OF NITRIC OXIDE FOR TREATING RESPIRATORY DISEASES | Advanced Inhalation Therapies (AIT) Ltd. | 7-
Mar- 12 |
7-
Mar -13 |
5-
Feb -15 |
| US20150031
038 |
SAMPLE PREPARATION METHODS | Not Available | 6-
Sep- 11 |
6-
Sep -12 |
29-
Jan- 15 |
| US20150031
016 |
Mixed Cell Diagnostic Systems For Detection Of Respiratory, Herpes and Enteric Viruses | Diagnostic Hybrids Inc. | 24-
Apr- 98 |
4-
Aug -14 |
29-
Jan- 15 |
| US20150031
014 |
DETECTING ANALYTES WITH A PH METER | The Board of Trustees of the University of Illinois | 25-
Jul-13 |
25-
Jul- 14 |
29-
Jan- 15 |
| US20150030
627 |
Trans-complementing, replication deficient lentiviral vectors and methods for making and using them | VIRxSYS.CON390 | 17-
Aug- 06 |
8-
Oct -14 |
29-
Jan- 15 |
| US20150030
626 |
IMMUNOMODULATORY CONJUGATES | Ascend Biopharamaceuticals Ltd | 9-
Nov- 11 |
9-
Nov -12 |
29-
Jan- 15 |
| US20150030
625 |
GAS57 MUTANT ANTIGENS AND GAS57 ANTIBODIES | Not Available | 12-
Sep- 07 |
13-
Oct -14 |
29-
Jan- 15 |
| US20150030
607 |
INFLUENZA HEMAGGLUTININ-SPECIFIC MONOCLONAL ANTIBODIES FOR PREVENTING AND TREATING INFLUENZA VIRUS INFECTION | Not Available | 20-
Oct- 10 |
9-
Oct -14 |
29-
Jan- 15 |
| US20150025
075 |
HETEROCYCLIC AMIDE DERIVATIVES AS P2X7 RECEPTOR ANTAGONISTS | ACTELION PHARMACEUTICALS LTD. | 20-
Jan- 12 |
18-
Jan -13 |
22-
Jan- 15 |
| US20150024
415 |
DETECTION AND QUANTIFICATION OF ANALYTES BASED ON SIGNAL INDUCED BY ALKALINE PHOSPHATE | The Board of Trustees of the University of Illinois | 16-
Feb- 12 |
19-
Feb -13 |
22-
Jan- 15 |
| US20150024
405 |
ENHANCED DEPOSITION OF CHROMOGENS UTILIZING PYRIMIDINE ANALOGS | Ventana Medical Systems, Inc. | 30-
Dec- 10 |
6-
Oct -14 |
22-
Jan- 15 |
| US20150024
002 |
ALPHAVIRUS VECTORS FOR RESPIRATORY PATHOGEN VACCINES | Not Available | 21-
May- 04 |
20-
Aug -14 |
22-
Jan- 15 |
| US20150023
990 |
ALKOXY SUBSTITUTED IMIDAZOQUINOLINES | 3M INNOVATIVE PROPERTIES COMPANY | 3-
Oct- 03 |
30-
Sep -14 |
22-
Jan- 15 |
| US20150023
924 |
VARIANT AAV AND COMPOSITIONS, METHODS AND USES FOR GENE TRANSFER TO CELLS, ORGANS AND TISSUES | Not Available | 22-
Jul-13 |
22-
Jul- 14 |
22-
Jan- 15 |
| US20150023
921 |
HEPATITIS C ANTIVIRAL COMPOSITIONS AND METHODS | BIOTRON LIMITED | 3-
Aug- 07 |
3-
Apr -14 |
22-
Jan- 15 |
| US20150023
879 |
DIAGNOSTIC CHEWING GUM FOR PATHOGENS | Julius-Maximilians-Universitaet Wuerzburg | 8-
Mar- 12 |
8-
Mar -13 |
22-
Jan- 15 |
| US20150021
204 |
AMPEROMETRIC GAS SENSOR | Not Available | 25-
Jun- 12 |
30-
Sep -14 |
22-
Jan- 15 |
| US20150021
203 |
AMPEROMETRIC GAS SENSOR | Not Available | 25-
Jun- 12 |
30-
Sep -14 |
22-
Jan- 15 |
| US20150018
396 |
PREVENTION AND TREATMENT OF RESPIRATORY INFECTION WITH PEROXISOME PROLIFERATOR ACTIVATOR RECEPTOR DELTA AGONIST | Not Available | 8-
Mar- 12 |
3-
Mar -13 |
15-
Jan- 15 |
| US20150018
332 |
Nuclear Transport Modulators and Uses Thereof | KARYOPHARM THERAPEUTICS INC. | 29-
Jul-11 |
26-
Jul- 12 |
15-
Jan- 15 |
| US20150017
251 |
MICROPARTICLES FOR USE IN IMMUNOGENIC COMPOSITIONS | NOVARTIS AG | 6-
Aug- 08 |
18-
Jul- 14 |
15-
Jan- 15 |
| US20150011
615 |
Carbohydrate Conjugates as Delivery Agents for Oligonucleotides | Not Available | 4-
Dec- 07 |
11-
Jul- 14 |
8-
Jan- 15 |
| US20150011
508 |
COMPOUNDS AND COMPOSITIONS AS C-KIT KINASE INHIBITORS | IRM LLC | 1-
Sep- 11 |
28-
Aug -12 |
8-
Jan- 15 |
| US20150010
983 |
COMPOSITIONS, METHODS AND USES FOR INDUCING VIRAL GROWTH | Not Available | 5-
Dec- 08 |
19-
Sep -14 |
8-
Jan- 15 |
| US20150005
227 |
METHOD OF TREATING AN ISCHEMIA-REPERFUSION INJURY- RELATED DISORDER BY ADMINISTERING GPCR LIGANDS | Not Available | 18-
Sep- 06 |
7-
Sep -14 |
1-
Jan- 15 |
| US20150004
188 |
COMPOSITIONS, COMPRISING IMPROVED IL-12 GENETIC CONSTRUCTS AND VACCINES, IMMUNOTHERAPEUTICS AND METHODS OF USING THE SAME | Not Available | 12-
Dec- 11 |
11-
Dec -12 |
1-
Jan- 15 |
| US20140364
492 |
PHORBOL TYPE DITERPENE COMPOUND, PHARMACEUTICAL COMPOSITION FOR TREATMENT OR PREVENTION OF VIRAL INFECTIOUS DISEASES INCLUDING SAME | KOREA RESEARCH INSTITUTE OF BIOSCIENCE AND BIOTECHNOLOGY | 26-
Oct- 11 |
28-
Sep -12 |
11-
Dec -14 |
| US20140364
408 |
Hydrazide Containing Nuclear Transport Modulators And Uses Thereof | KARYOPHARM THERAPEUTICS INC. | 29-
Jul-11 |
29-
Jul- 12 |
11-
Dec -14 |
| US20140364
358 |
Method for Preventing and Treating Hyperpermeability | Apeptico Forschung UND Entwicklung GMBH | 5-
Mar- 09 |
7-
Mar -14 |
11-
Dec -14 |
| US20140363
465 |
MYCOBACTERIAL VACCINE VECTORS AND METHODS OF USING THE SAME | Beth Israel Deaconess Medical Center, Inc. | 4-
Apr- 11 |
4-
Apr -12 |
11-
Dec -14 |
| US20140363
422 |
METHOD OF PROVIDING MONOCLONAL AUTO-ANTIBODIES WITH DESIRED SPECIFICITY | Not Available | 28-
Dec- 11 |
2-
Jan -13 |
11-
Dec -14 |
| US20140357
673 |
Deubiquitinase Inhibitors and Methods for Use of the Same | Not Available | 24-
Sep- 10 |
31-
Jul- 14 |
4-
Dec -14 |
| US20140356
390 |
MODULATION OF REPLICATIVE FITNESS BY DEOPTIMIZATION OF SYNONYMOUS CODONS | The Government of the United States of America as represented by the Secretary of the Department of | 8-
Oct- 04 |
20-
Aug -14 |
4-
Dec -14 |
| US20140349
375 |
METHYLSULFONYLMETHANE (MSM) TO MODULATE MICROBIAL ACTIVITY | Biogentic Innovations, LLC | 30-
Oct- 09 |
12-
Aug -14 |
27-
Nov -14 |
| US20140349
295 |
METHOD FOR DETECTING TARGET NUCLEIC ACID | Not Available | 31-
Oct- 11 |
25-
Oct -12 |
27-
Nov -14 |
| US20140349
276 |
SIGNAL PROPAGATION BIOMOLECULES, DEVICES AND METHODS | STC.UNM | 21-
May- 13 |
21-
May -14 |
27-
Nov -14 |
| US20140348
906 |
IMMUNOSTIMULATORY COMPOSITIONS COMPRISING LIPOSOME- ENCAPSULATED OLIGONUCLEOTIDES AND EPITOPES | Not Available | 17-
Jul-09 |
13-
Aug -14 |
27-
Nov -14 |
| US20140348
791 |
MODIFIED ADENOVIRAL VECTORS AND METHODS OF TREATMENT USING SAME | Beth Israel Deaconess Medical Center, Inc. | 9-
Sep- 11 |
7-
Sep -12 |
27-
Nov -14 |
| US20140348
785 |
NEUTRALIZING GP41 ANTIBODIES AND THEIR USE | Not Available | 7-
Nov- 11 |
7-
Nov -12 |
27-
Nov -14 |
| US20140348
781 |
CONJUGATES OF GM-CSF AND IL-9, COMPOSITIONS AND METHODS RELATED THERETO | CHILDREN’S HEALTHCARE OF ATLANTA, INC | 22-
May- 13 |
20-
May -14 |
27-
Nov -14 |
| US20140342
941 |
METHOD FOR USING PERMUTED NUCLEIC ACID PROBES | Ventana Medical Systems, Inc. | 1-
Sep- 06 |
5-
Aug -14 |
20-
Nov -14 |
| US20140342
407 |
NEUTRALIZING GP41 ANTIBODIES AND THEIR USE | The United States of America, as represented by the Secretary, Department of Health and Human Serv | 7-
Nov- 11 |
4-
Aug -14 |
20-
Nov -14 |
| US20140336
245 |
VIRUS VECTORS FOR HIGHLY EFFICIENT TRANSGENE DELIVERY | Not Available | 22-
Nov- 11 |
21-
Nov -12 |
13-
Nov -14 |
| US20140335
618 |
TAL EFFECTOR-MEDIATED DNA MODIFICATION | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
14-
Apr -14 |
13-
Nov -14 |
| US20140335
592 |
TAL EFFECTOR-MEDIATED DNA MODIFICATION | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
24-
Feb -14 |
13-
Nov -14 |
| US20140335
117 |
Identification and Attenuation of the Immunosuppressive Domains in Fusion Proteins of Enveloped RNA Viruses | Not Available | 7-
Oct- 11 |
5-
Oct -12 |
13-
Nov -14 |
| US20140335
111 |
VACCINES AND IMMUNOTHERAPEUTICS USING IL-28 AND COMPOSITIONS AND METHODS OF USING THE SAME | The Trustees of the University of Pennsylvania | 4-
Apr- 08 |
28-
Apr -14 |
13-
Nov -14 |
| US20140335
104 |
Methods of Detecting Cells with a Disrupted Cell Membrane, Cells Infected with A Pathogen, Dying Cells or Dead Cells | Not Available | 13-
May- 08 |
14-
Apr -14 |
13-
Nov -14 |
| US20140329
889 |
CYCLIC DI-NUCLEOTIDE INDUCTION OF TYPE I INTERFERON | Not Available | 3-
May- 13 |
2-
May -14 |
6-
Nov -14 |
| US20140329
878 |
OLIGONUCLEOTIDE MODULATORS OF THE TOLL-LIKE RECEPTOR PATHWAY | Not Available | 3-
Mar- 11 |
1-
Mar -12 |
6-
Nov -14 |
| US20140329
817 |
MYXOVIRUS THERAPEUTICS, COMPOUNDS, AND USES RELATED THERETO | CHILDREN’S HEATLHCARE OF ATLANTA, INC. | 24-
Oct- 11 |
24-
Oct -12 |
6-
Nov -14 |
| US20140328
946 |
ANTIMICROBIAL SOLUTIONS CONTAINING DICHLORIDE MONOXIDE AND METHODS OF MAKING AND USING THE SAME | Not Available | 13-
Mar- 07 |
21-
Jul- 14 |
6-
Nov -14 |
| US20140328
874 |
SAMPLE QUANTIFICATION BY DISC CENTRIFUGATION | Not Available | 19-
Oct- 11 |
19-
Oct -12 |
6-
Nov -14 |
| US20140323
556 |
SCALABLE MANUFACTURING PROCESS TO PRODUCE RECOMBINANT LENTIVIRAL VECTORS IN SERUM-FREE SUSPENSION CELL CULTURE SYSTEM | Not Available | 15-
Mar- 13 |
17-
Mar -14 |
30-
Oct- 14 |
| US20140323
392 |
METHODS AND COMPOSITIONS RELATED TO INHIBITION OF VIRAL ENTRY | UNIVERSITY OF UTAH RESEARCH FOUNDATION | 28-
Mar- 11 |
28-
Mar -12 |
30-
Oct- 14 |
| US20140323
390 |
COMPOUNDS AND COMPOSITIONS AS TLR2 AGONISTS | IRM LLC | 23-
Mar- 10 |
9-
Jul- 14 |
30-
Oct- 14 |
| US20140322
355 |
HETEROCYCLIC MODULATORS OF LIPID SYNTHESIS | 3-V BIOSCIENCES, INC. | 8-
Mar- 11 |
25-
Jun -14 |
30-
Oct- 14 |
| US20140317
766 |
ANTIBODY PRODUCING NON-HUMAN MAMMALS | Not Available | 27-
Jun- 08 |
29-
Apr -14 |
23-
Oct- 14 |
| US20140315
982 |
DOUBLE-STRANDED NUCLEIC ACID MOLECULE FOR GENE EXPRESSION CONTROL | Osaka City University | 2-
Nov- 11 |
1-
Nov -12 |
23-
Oct- 14 |
| US20140315
215 |
THERMOSTABLE ASSAY REAGENTS | The Secretary of State for Health | 14-
Sep- 11 |
14-
Sep -12 |
23-
Oct- 14 |
| US20140314
858 |
IPNV-ISAV BIVALENT VACCINE USING A VIRUS-LIKE PARTICLE- BASED PLATFORM AND METHODS OF USING THE SAME | Advanced BioNutrition Corporation | 9-
Sep- 11 |
7-
Sep -12 |
23-
Oct- 14 |
| US20140314
839 |
CONTROLLED-RELEASE PEPTIDE COMPOSITIONS AND USES THEREOF | Not Available | 2-
Dec- 11 |
30-
Nov -12 |
23-
Oct- 14 |
| US20140314
809 |
MALARIA ANTIGEN SCREENING METHOD | Not Available | 19-
Apr- 13 |
19-
Apr -13 |
23-
Oct- 14 |
| US20140314
797 |
YEAST STRAIN FOR THE PRODUCTION OF PROTEINS WITH TERMINAL ALPHA-1,3-LINKED GALACTOSE | Not Available | 30-
May- 08 |
2-
Jul- 14 |
23-
Oct- 14 |
| US20140314
755 |
ANTIBODY PRODUCING NON-HUMAN MAMMALS | Not Available | 27-
Jun- 08 |
30-
Apr -14 |
23-
Oct- 14 |
| US20140314
739 |
Vaccine adjuvant composition comprising inulin particles | Vaxine Pty Ltd. | 18-
Jun- 11 |
19-
Jun -12 |
23-
Oct- 14 |
| US20140309
189 |
HIGHLY ACTIVE NUCLEOSIDE DERIVATIVE FOR THE TREATMENT OF HCV | Achillion Pharmaceuticals, Inc. | 12-
Apr- 13 |
14-
Apr -14 |
16-
Oct- 14 |
| US20140309
164 |
DEUTERATED NUCLEOSIDE PRODRUGS USEFUL FOR TREATING HCV | Achillion Pharmaceuticals, Inc. | 12-
Apr- 13 |
14-
Apr -14 |
16-
Oct- 14 |
| US20140308
379 |
USE OF FLAXSEED AND FLAXSEED DERIVATIVES FOR TREATMENT OF NEUROLOGICAL DISORDERS AND VIRAL DISEASES | The Trustees of the University of Pennsylvania | 20-
Nov- 12 |
19-
Nov -13 |
16-
Oct- 14 |
| US20140308
313 |
COMPOSITIONS AND METHODS FOR IMMUNISATION USING CD1D LIGANDS | Not Available | 15-
Mar- 06 |
26-
Jun -14 |
16-
Oct- 14 |
| US20140308
211 |
MUTANT PROTEASE BIOSENSORS WITH ENHANCED DETECTION CHARACTERISTICS | Not Available | 11-
May- 10 |
5-
May -14 |
16-
Oct- 14 |
| US20140303
232 |
LIPIDS AND LIPID COMPOSITIONS FOR THE DELIVERY OF ACTIVE AGENTS | NOVARTIS AG | 8-
Mar- 13 |
7-
Mar -14 |
9-
Oct- 14 |
| US20140303
071 |
CYCLIC ANTIMICROBIAL PEPTIDES | Not Available | 22-
Dec- 05 |
20-
Jun -14 |
9-
Oct- 14 |
| US20140302
124 |
Cytotoxic T Lymphocyte Inducing Immunogens For Prevention Treatment and Diagnosis of INFLUENZA VIRUS INFECTION | Immunotape, Inc. | 19-
Oct- 11 |
18-
Oct -12 |
9-
Oct- 14 |
| US20140302
120 |
CONJUGATES OF SYNTHETIC TLR AGONISTS AND USES THEREFOR | Not Available | 7-
Feb- 07 |
19-
Jun -14 |
9-
Oct- 14 |
| US20140302
091 |
METHODS AND COMPOSITIONS FOR LIVE ATTENUATED VIRUSES | Not Available | 6-
Apr- 07 |
18-
Nov -11 |
9-
Oct- 14 |
| US20140302
077 |
LIVE ATTENUATED INFLUENZA VIRUS | Westfaelische Wilhelms-Universitaet Munester | 2-
Sep- 11 |
28-
Aug -12 |
9-
Oct- 14 |
| US20140298
500 |
MUTANT PROTEASE BIOSENSORS WITH ENHANCED DETECTION CHARACTERISTICS | Not Available | 11-
May- 10 |
5-
May -14 |
2-
Oct- 14 |
| US20140296
179 |
NUTRITIONAL COMPOSITION COMPRISING IMMUNOGLOBULINS AND OLIGOSACCHARIDES | N.V. NUTRICIA | 24-
Aug- 04 |
5-
Mar -14 |
2-
Oct- 14 |
| US20140295
404 |
SAMPLE FIXATION AND STABILISATION | Not Available | 1-
Mar- 13 |
28-
Feb -14 |
2-
Oct- 14 |
| US20140294
828 |
ALPHABODIES SPECIFICALLY BINDING TO VIRAL PROTEINS AND METHODS FOR PRODUCING THE SAME | COMPLIX SA | 6-
Jan- 11 |
6-
Jan -11 |
2-
Oct- 14 |
| US20140294
765 |
LSR ANTIBODIES, AND USES THEREOF FOR TREATMENT OF CANCER | COMPUGEN LTD. | 21-
Jun- 12 |
19-
Jun -13 |
2-
Oct- 14 |
| US20140294
727 |
PEPTIDES FOR ASSISTING DELIVERY ACROSS THE BLOOD BRAIN BARRIER | Children’s Medical Center Corporation | 22-
May- 06 |
30-
Apr -14 |
2-
Oct- 14 |
| US20140294
670 |
TRANSPORTABLE VACUUM ASSISTED DECONTAMINATION UNIT AND DECONTAMINATION PROCESS | STERIS Inc. | 1-
Apr- 13 |
31-
Mar -14 |
2-
Oct- 14 |
| US20140287
987 |
METHODS AND COMPOSITIONS FOR THE TREATMENT OF CANCER OR OTHER DISEASES | CITY OF HOPE | 26-
Jan- 07 |
9-
Jun -14 |
25-
Sep -14 |
| US20140286
988 |
HYDRAZINO 1H-IMIDAZOQUINOLIN-4-AMINES AND CONJUGATES MADE THEREFROM | 3M Innovative Properties Company | 3-
Jun- 11 |
1-
Jun -12 |
25-
Sep -14 |
| US20140283
945 |
LOADING VIALS | BioFire Diagnostics, LLC | 10-
Nov- 11 |
9-
Nov -12 |
25-
Sep -14 |
| US20140275
237 |
BERAPROST ISOMER AS AN AGENT FOR THE TREATMENT OF VIRAL INFECTION | Gemmus Pharma Inc. | 15-
Mar- 13 |
12-
Mar -14 |
18-
Sep -14 |
| US20140275
159 |
SUBSTITUTED 2-AZA-BICYCLO[2.2.2]OCTANE-3-CARBOXYLIC ACID (BENZYL-CYANO-METHYL)-AMIDES INHIBITORS OF CATHEPSIN C | BOEHRINGER INGELHEIM INTERNATIONAL GMBH | 14-
Mar- 13 |
12-
Mar -14 |
18-
Sep -14 |
| US20140275
155 |
SUBSTITUTED BICYCLIC 1-CARBOXYLIC-ACID (BENZYL-CYANO- METHYL)-AMIDES INHIBITORS OF CATHEPSIN C | BOEHRINGER INGELHEIM INTERNATIONAL GMBH | 14-
Mar- 13 |
12-
Mar -14 |
18-
Sep -14 |
| US20140275
114 |
SUBSTITUTED 2-AZA-BICYCLO[2.2.1]HEPTANE-3-CARBOXYLIC ACID (CYANO-METHYL)-AMIDES INHIBITORS OF CATHEPSIN C | BOEHRINGER INGELHEIM INTERNATIONAL GMBH | 14-
Mar- 13 |
12-
Mar -14 |
18-
Sep -14 |
| US20140275
037 |
SULFONYL SEMICARBAZIDES, SEMICARBAZIDES AND UREAS, PHARMACEUTICAL COMPOSITIONS THEREOF, AND METHODS FOR TREATING HEMORRHAGIC FEVER VIRUSES, INCLUDING INFECTIONS ASSOCIATED WITH ARENAVIRUSES | Siga Technologies, Inc. | 6-
Dec- 04 |
19-
Dec -13 |
18-
Sep -14 |
| US20140275
025 |
SUBSTITUTED 2-AZA-BICYCLO[2.2.1]HEPTANE-3-CARBOXYLIC ACID (BENZYL-CYANO-METHYL)-AMIDES INHIBITORS OF CATHEPSIN C | BOEHRINGER INGELHEIM INTERNATIONAL GMBH | 14-
Mar- 13 |
12-
Mar -14 |
18-
Sep -14 |
| US20140274
980 |
COMPOUND | RESPIVERT LTD. | 15-
Mar- 13 |
14-
Mar -14 |
18-
Sep -14 |
| US20140273
228 |
METHOD FOR PROPAGATING ADENOVIRAL VECTORS ENCODING INHIBITORY GENE PRODUCTS | GenVec, Inc. | 10-
Nov- 05 |
28-
May -14 |
18-
Sep -14 |
| US20140273
156 |
LUCIFERASE BIOSENSOR | PROMEGA CORPORATION | 10-
Oct- 03 |
14-
Feb -14 |
18-
Sep -14 |
| US20140271
829 |
RECOMBINANT SELF-REPLICATING POLYCISTRONIC RNA MOLECULES | Not Available | 11-
Oct- 11 |
11-
Oct -12 |
18-
Sep -14 |
| US20140271
700 |
COMPOSITIONS AND METHODS FOR TREATING CLOSTRIDIUM DIFFICILE-ASSOCIATED DISEASES | National Health Research Institutes | 14-
Mar- 13 |
13-
Mar -14 |
18-
Sep -14 |
| US20140271
580 |
IMMUNOPROTECTIVE PRIMARY MESENCHYMAL STEM CELLS AND METHODS | THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND | 14-
Mar- 13 |
14-
Mar -13 |
18-
Sep -14 |
| US20140271
550 |
Constructs and Methods for Delivering Molecules via Viral Vectors with Blunted Innate Immune Responses | The Trustees of the University of Pennsylvania | 14-
Mar- 13 |
14-
Mar -14 |
18-
Sep -14 |
| US20140270
457 |
STAIN-FREE HISTOPATHOLOGY BY CHEMICAL IMAGING | The Board of Trustees of the University of Illinois | 15-
Mar- 13 |
14-
Mar -14 |
18-
Sep -14 |
| US20140255
472 |
PEGYLATED LIPOSOMES FOR DELIVERY OF IMMUNOGEN-ENCODING RNA | Not Available | 31-
Aug- 11 |
31-
Aug -12 |
11-
Sep -14 |
| US20140255
439 |
VIRUS-LIKE PARTICLES AND PROCESS FOR PREPARING SAME | FOLIA BIOTECH INC. | 13-
May- 11 |
1-
May -12 |
11-
Sep -14 |
| US20140255
426 |
WNT PATHWAY INHIBITORS FOR TREATING VIRAL INFECTIONS | Children’s Healthcare of Atlanta, Inc. | 11-
Mar- 13 |
10-
Mar -14 |
11-
Sep -14 |
| US20140255
349 |
PEPTIDES WITH VIRAL INFECTION ENHANCING PROPERTIES AND THEIR USE | Centre National de la Recherche Scientique | 30-
Jun- 11 |
28-
Jun -12 |
11-
Sep -14 |
| US20140249
296 |
USING SORTASES TO INSTALL CLICK CHEMISTRY HANDLES FOR PROTEIN LIGATION | Whitehead Institute for Biomedical Research | 28-
Jun- 11 |
28-
Jun -12 |
4-
Sep -14 |
| US20140249
129 |
Substituted Bicyclic Dihydropyrimidinones And Their Use As Inhibitors Of Neutrophil Elastase Activity | Boehringer Ingelheim International GmbH | 4-
Mar- 13 |
20-
Feb -14 |
4-
Sep -14 |
| US20140248
620 |
Methods and Compositions for Prostate Cancer Metastasis | Florida Agricultural and Mechanical University (FAMU) | 25-
Mar- 11 |
23-
Mar -12 |
4-
Sep -14 |
| US20140248
357 |
Respiratory Disease Treatment | PULMAGEN THERAPEUTICS (INFLAMMATION) LIMITED | 7-
Aug- 08 |
7-
Apr -14 |
4-
Sep -14 |
| US20140248
320 |
ADJUVANTED INFLUENZA B VIRUS VACCINES FOR PEDIATRIC PRIMING | Not Available | 20-
Oct- 11 |
19-
Oct -12 |
4-
Sep -14 |
| US20140248
313 |
COMBINATION ADJUVANT FORMULATION | Dalhousie University | 16-
Oct- 08 |
15-
Feb -13 |
4-
Sep -14 |
| US20140248
312 |
INFLUENZA VACCINES INCLUDING COMBINATIONS OF PARTICULATE ADJUVANTS AND IMMUNOPOTENTIATORS | NOVARTIS VACCINES AND DIAGNOSTICS SRL | 4-
Nov- 05 |
28-
Feb -14 |
4-
Sep -14 |
| US20140243
505 |
BISPECIFIC ANTIBODY | WUHAN YZY BIOPHARMA CO., LTD. | 21-
Nov- 12 |
13-
Mar -14 |
28-
Aug -14 |
| US20140243
341 |
BROAD-SPECTRUM ANTIVIRALS AGAINST 3C OR 3C-LIKE PROTEASES OF PICORNAVIRUS-LIKE SUPERCLUSTER: PICORNAVIRUSES, CALICIVIRUSES AND CORONAVIRUSES | Not Available | 27-
Sep- 11 |
27-
Sep -12 |
28-
Aug -14 |
| US20140242
692 |
MAMMALIAN GENES INVOLVED IN TOXICITY AND INFECTION | Not Available | 10-
Dec- 10 |
11-
Dec -11 |
28-
Aug -14 |
| US20140242
152 |
IMMUNOGENIC COMPOSITIONS AND USES THEREOF | Not Available | 6-Jul-
11 |
6-
Jul- 12 |
28-
Aug -14 |
| US20140242
108 |
Conjugated TLR7 and/or TLR8 and TLR2 polycationic agonists | CAYLA | 22-
Feb- 13 |
22-
Feb -13 |
28-
Aug -14 |
| US20140235
844 |
Short Interfering RNA (siRNA) Analogues | Santaris Pharma A/S | 21-
Mar- 03 |
11-
Feb -14 |
21-
Aug -14 |
| US20140235
653 |
HYDRAZIDE CONTAINING NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Karyopharm Therapeutics, Inc. | 29-
Jul-11 |
19-
Mar -14 |
21-
Aug -14 |
| US20140235
460 |
QUANTITATIVE NUCLEASE PROTECTION ASSAY (QNPA) AND SEQUENCING (QNPS) IMPROVEMENTS | HTG Molecular Diagnostics, Inc. | 4-
May- 11 |
1-
May -14 |
21-
Aug -14 |
| US20140234
845 |
ORGANISM IDENTIFICATION PANEL | Not Available | 2-
Apr- 07 |
1-
Apr -08 |
21-
Aug -14 |
| US20140234
374 |
COMPOSITIONS AND METHODS FOR ACTIVATING INNATE AND ALLERGIC IMMUNITY | ID BIOMEDICAL CORPORATION OF QUEBEC | 26-
Apr- 12 |
28-
Apr -14 |
21-
Aug -14 |
| US20140234
372 |
NOVEL VLPS DERIVED FROM CELLS THAT DO NOT EXPRESS A VIRAL MATRIX OR CORE PROTEIN | Not Available | 25-
May- 07 |
14-
Apr -14 |
21-
Aug -14 |
| US20140234
274 |
Directed Evolution and In Vitro Panning of Virus Vectors | University of North Carolina at Chapel Hill | CO i i | 17-
Jan -14 |
21-
Aug -14 |
| US20140228
347 |
COMPOUNDS AND COMPOSITIONS AS C-KIT KINASE INHIBITORS | IRM LLC | 1-
Sep- 11 |
22-
Apr -14 |
14-
Aug -14 |
| US20140227
704 |
METHODS FOR RAPID IDENTIFICATION AND QUANTITATION OF NUCLEIC ACID VARIANTS | IBIS BIOSCIENCES, INC. | 11-
Nov- 09 |
7-
Oct -13 |
14-
Aug -14 |
| US20140227
346 |
IMMUNOGENIC COMBINATION COMPOSITIONS AND USES THEREOF | Not Available | 6-Jul-
11 |
6-
Jul- 12 |
14-
Aug -14 |
| US20140227
317 |
HETEROBIFUNCTIONAL LINKERS WITH POLYETHYLENE GLYCOL SEGMENTS AND IMMUNE RESPONSE MODIFIER CONJUGATES MADE THEREFROM | 3M INNOVATIVE PROPERTIES COMPANY | 3-
Jun- 11 |
1-
Jun -12 |
14-
Aug -14 |
| US20140227
262 |
PD-1 Antagonists and Methods for Treating Infectious Disease | AMPLIMMUNE, INC. | 25-
Aug- 08 |
1-
Nov -13 |
14-
Aug -14 |
| US20140221
631 |
BORON-CONTAINING SMALL MOLECULES | Anacor Pharmaceuticals, Inc. | 16-
Feb- 06 |
7-
Mar -14 |
7-
Aug -14 |
| US20140221
394 |
HYDROBENZAMIDE DERIVATIVES AS INHIBITORS OF HSP90 | Astex Therapeutic Ltd. | 12-
Oct- 06 |
7-
Jan -14 |
7-
Aug -14 |
| US20140221
335 |
SUBSTITUTED BICYCLIC DIHYDROPYRIMIDINONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | BOEHRINGER INGELHEIM INTERNATIONAL GMBH | 6-
Feb- 13 |
28-
Jan -14 |
7-
Aug -14 |
| US20140220
690 |
MAMMALIAN GENES INVOLVED IN INFECTION | VANDERBILT UNIVERSITY | 11-
Nov- 09 |
28-
Oct -10 |
7-
Aug -14 |
| US20140220
573 |
EMBODIMENTS OF A PROBE AND METHOD FOR TARGETING NUCLEIC ACIDS | University Of Idaho | 19-
Jul-11 |
19-
Jul- 12 |
7-
Aug -14 |
| US20140220
086 |
Antimicrobial Compositions and Methods of Use Thereof | Not Available | 21-
Sep- 11 |
21-
Sep -12 |
7-
Aug -14 |
| US20140220
083 |
CATIONIC OIL-IN-WATER EMULSIONS | Not Available | 6-Jul-
11 |
6-
Jul- 12 |
7-
Aug -14 |
| US20140220
063 |
VACCINE COMPOSITION | Not Available | 5-
Feb- 13 |
29-
Jan -14 |
7-
Aug -14 |
| US20140212
498 |
OIL-IN-WATER EMULSIONS THAT CONTAIN NUCLEIC ACIDS | Not Available | 6-Jul-
11 |
6-
Jul- 12 |
31-
Jul- 14 |
| US20140212
429 |
BINDING MEMBERS-513 | MEDIMMUNE LIMITED | 7-
Nov- 08 |
16-
Apr -14 |
31-
Jul- 14 |
| US20140206
682 |
COMPOUNDS AND COMPOSITIONS AS PDGFR KINASE INHIBITORS | IRM LLC | 1-
Sep- 11 |
31-
Aug -12 |
24-
Jul- 14 |
| US20140206
556 |
Systems and Methods for Identifying Replikin Scaffolds and Uses of Said Replikin Scaffolds | Not Available | 6-
Jun- 03 |
19-
Jun -13 |
24-
Jul- 14 |
| US20140205
629 |
TC-83-DERIVED ALPHAVIRUS VECTORS, PARTICLES AND METHODS | AlphaVax, Inc. | 18-
May- 04 |
28-
Mar -14 |
24-
Jul- 14 |
| US20140203
192 |
SYSTEMS AND METHODS FOR DISTINGUISHING OPTICAL SIGNALS OF DIFFERENT MODULATION FREQUENCIES IN AN OPTICAL SIGNAL DETECTOR | Gen-Probe Incorporated | 24-
Feb- 11 |
21-
Mar -14 |
24-
Jul- 14 |
| US20140203
189 |
SYSTEMS AND METHODS FOR DISTINGUISHING OPTICAL SIGNALS OF DIFFERENT MODULATION FREQUENCIES IN AN OPTICAL SIGNAL DETECTOR | GEN-PROBE INCORPORATED | 24-
Feb- 11 |
21-
Mar -14 |
24-
Jul- 14 |
| US20140200
149 |
Methods and compositions for identification of source of microbial contamination in a sample | THE REGENTS OF THE UNIVERSITY OF CALIFORNIA | 6-
Mar- 12 |
6-
Mar -13 |
17-
Jul- 14 |
| US20140199
379 |
COMPOSITIONS HAVING MEANS FOR TARGETING AT LEAST ONE ANTIGEN TO DENDRITIC CELLS | ASSISTANCE PUBLIQUE – HOPITAUX DE PARIS | 22-
Jul-11 |
23-
Jul- 12 |
17-
Jul- 14 |
| US20140199
347 |
ADJUVANT COMPOSITIONS WITH 4-1BBL | UNIVERSITY OF LOUISVILLE RESEARCH FOUNDATION, INC. | 10-
Feb- 11 |
7-
Feb -12 |
17-
Jul- 14 |
| US20140199
281 |
SYNERGISTIC BACTERIAL COMPOSITIONS AND METHODS OF PRODUCTION AND USE THEREOF | Seres Health, Inc. | 23-
Nov- 12 |
20-
Mar -14 |
17-
Jul- 14 |
| US20140194
628 |
SUBSTITUTED IMIDAZO RING SYSTEMS AND METHODS | 3M INNOVATIVE PROPERTIES COMPANY | 25-
Nov- 03 |
11-
Mar -14 |
10-
Jul- 14 |
| US20140194
500 |
Methods For Treating of SARS | Not Available | 8-
Jan- 13 |
8-
Jan -13 |
10-
Jul- 14 |
| US20140194
345 |
NOVEL DEPSIPEPTIDE AND USES THEREOF | Not Available | 3-
Dec- 12 |
3-
Dec -13 |
10-
Jul- 14 |
| US20140193
804 |
Biological Specimen Collection and Transport System and Method of Use | Longhorn Vaccines and Diagnostics, LLC | 1-
Oct- 07 |
7-
Jan -14 |
10-
Jul- 14 |
| US20140193
439 |
VACCINES AND IMMUNOTHERAPEUTICS COMPRISING IL-15 RECEPTOR ALPHA AND/OR NUCLEIC ACID MOLECULES ENCODING THE SAME, AND METHODS FOR USING THE SAME | Not Available | 14-
Sep- 09 |
6-
Jan -14 |
10-
Jul- 14 |
| US20140186
396 |
METHODS FOR STABILIZING INFLUENZA ANTIGEN ENVELOPED VIRUS-BASED VIRUS-LIKE PARTICLE SOLUTIONS | TAKEDA VACCINES, INC. | 28-
Dec- 09 |
6-
Mar -14 |
3-
Jul- 14 |
| US20140186
292 |
DEFECTIVE RIBOSOMAL PRODUCTS IN BLEBS (DRIBBLES) AND METHODS OF USE TO STIMULATE AN IMMUNE RESPONSE | Providence Health & Services – Oregon | 29-
Jul-05 |
10-
Feb -14 |
3-
Jul- 14 |
| US20140179
761 |
TARGETING LIPIDS | Tekmira Pharmaceuticals Corporation | 4-
Dec- 08 |
22-
Oct -13 |
26-
Jun- 14 |
| US20140179
714 |
NOVEL COMPOUNDS | Chiesi Farmaceutici S.p.A. | 14-
Sep- 11 |
11-
Feb -14 |
26-
Jun- 14 |
| US20140178
977 |
SYSTEMS AND METHODS FOR DETECTING MULTIPLE OPTICAL SIGNALS | Gen-Probe Incorporated | 10-
Mar- 05 |
3-
Mar -14 |
26-
Jun- 14 |
| US20140178
429 |
Vaccines Including Antigen From Four Strains of Influenza Virus | NOVARTIS AG | 6-
Dec- 06 |
24-
Sep -13 |
26-
Jun- 14 |
| US20140178
428 |
QUALITY CONTROL METHODS FOR OIL-IN-WATER EMULSIONS CONTAINING SQUALENE | Novartis AG | 8-
Nov- 06 |
27-
Aug -13 |
26-
Jun- 14 |
| US20140178
422 |
PRIMARY MESENCHYMAL STEM CELLS AS A VACCINE PLATFORM | The Administrators of the Tulane Educational Fund | 21-
Dec- 12 |
15-
Nov -13 |
26-
Jun- 14 |
| US20140171
447 |
CHLORO-PYRAZINE CARBOXAMIDE DERIVATIVES WITH EPITHELIAL
SODIUM CHANNEL BLOCKING ACTIVITY |
Parion Sciences, Inc. | 17-
Dec- 12 |
13-
Dec -13 |
19-
Jun- 14 |
| US20140171
414 |
NOVEL COMPOUNDS | CHIESI FARMACEUTICI S.p.A. | 18-
Dec- 12 |
17-
Dec -13 |
19-
Jun- 14 |
| US20140170
709 |
VECTOR FOR GENE THERAPY | TAKARA BIO INC. | 20-
Apr- 07 |
31-
Jan -14 |
19-
Jun- 14 |
| US20140170
244 |
3,5-DIAMINO-6-CHLORO-N-(N-(4-PHENYLBUTYL)CARBAMIMIDOYL) PYRAZINE-2- CARBOXAMIDE COMPOUNDS | Parion Sciences, Inc. | 17-
Dec- 12 |
13-
Dec -13 |
19-
Jun- 14 |
| US20140170
183 |
D-Amino Acid Derivative-Modified Peptidoglycan and Methods of Use Thereof | The Regents of the University of California | 30-
Nov- 12 |
27-
Nov -13 |
19-
Jun- 14 |
| US20140170
174 |
POLYCHLORINATED BIPHENYLS AND SQUALENE-CONTAINING ADJUVANTS | Novartis AG | 28-
Dec- 07 |
10-
Dec -13 |
19-
Jun- 14 |
| US20140170
156 |
HUMAN MONOCLONAL ANTIBODIES AGAINST INTERLEUKIN 8 (IL-8) | GENMAB A/S | 16-
Dec- 02 |
7-
Nov -13 |
19-
Jun- 14 |
| US20140170
141 |
POLYPEPTIDES AND USES THEREOF FOR TREATMENT OF AUTOIMMUNE DISORDERS AND INFECTION | COMPUGEN LTD. | 30-
Jun- 11 |
1-
Jul- 12 |
19-
Jun- 14 |
| US20140163
213 |
CpG Oligonucleotide Analogs Containing Hydrophobic T Analogs with Enhanced Immunostimulatory Activity | COLEY PHARMACEUTICAL GMBH | 27-
Sep- 06 |
4-
Oct -13 |
12-
Jun- 14 |
| US20140163
035 |
HETEROCYCLIC AMIDE DERIVATIVES AS P2X7 RECEPTOR ANTAGONISTS | ACTELION PHARMACEUTICALS LTD. | 22-
Jul-11 |
20-
Jul- 12 |
12-
Jun- 14 |
| US20140162
941 |
PROCESS FOR PREPARING BIOLOGICAL SAMPLES | BAYLOR COLLEGE OF MEDICINE | 27-
Jul-11 |
26-
Jul- 12 |
12-
Jun- 14 |
| US20140162
888 |
CIRCULATING BIOMARKERS FOR DISEASE | Not Available | 6-
Apr- 10 |
6-
Apr -11 |
12-
Jun- 14 |
| US20140162
357 |
METHODS FOR GENERATION OF ANTIBODIES | NATIONAL JEWISH HEALTH | 13-
Mar- 07 |
4-
Sep -13 |
12-
Jun- 14 |
| US20140162
285 |
Methods of Modulating Vesicular Trafficking | The General Hospital Corporation | 21-
May- 09 |
2-
Dec -13 |
12-
Jun- 14 |
| US20140161
906 |
USE OF ANGIOGENESIS ANTAGONISTS IN CONDITIONS OF ABNORMAL VENOUS PROLIFERATION | UNIVERSITY OF UTAH RESEARCH FOUNDATION | 8-
Nov- 07 |
12-
Jun -13 |
12-
Jun- 14 |
| US20140161
724 |
Compositions and Imaging Methods Comprising Detectably Labeled Phosphatidylethanolamine-Binding Peptides | Board of Regents, The University of Texas System | 15-
Jul-02 |
16-
Dec -13 |
12-
Jun- 14 |
| US20140155
370 |
NUCLEAR TRANSPORT MODULATORS AND USES THEREOF | Not Available | 5-
Mar- 10 |
28-
Jun -13 |
5-
Jun- 14 |
| US20140154
288 |
PAPAYA MOSAIC VIRUS COMPOSITIONS AND USES THEREOF FOR STIMULATION OF THE INNATE IMMUNE RESPONSE | FOLIA BIOTECH INC. | 13-
May- 11 |
1-
May -12 |
5-
Jun- 14 |
| US20140154
282 |
CONJUGATES UTILIZING PLATFORM TECHNOLOGY FOR STIMULATING IMMUNE RESPONSE | Not Available | 26-
Jan- 11 |
26-
Jan -12 |
5-
Jun- 14 |
| US20140154
254 |
HETERODIMERIC IMMUNOGLOBULINS | AMGEN INC. | 21-
Nov- 12 |
21-
Nov -13 |
5-
Jun- 14 |
| US20140148
350 |
CIRCULATING BIOMARKERS FOR DISEASE | Not Available | 18-
Aug- 10 |
18-
Aug -11 |
29-
May -14 |
| US20140142
118 |
CHEMICALLY AND METABOLICALLY STABLE DIPEPTIDE POSSESSING POTENT SODIUM CHANNEL BLOCKER ACTIVITY | PARION SCIENCES, INC. | 27-
Jun- 11 |
27-
Jun -12 |
22-
May -14 |
| US20140142
064 |
BORON-CONTAINING SMALL MOLECULES | ANACOR PHARMACEUTICALS, INC. | 16-
Feb- 05 |
27-
Jan -14 |
22-
May -14 |
| US20140141
986 |
CIRCULATING BIOMARKERS | Not Available | 22-
Feb- 11 |
17-
Feb -12 |
22-
May -14 |
| US20140141
502 |
Methods For Concurrent Identification And Quantification Of An Unknown Bioagent | IBIS BIOSCIENCES, INC. | 18-
Feb- 04 |
27-
Jan -14 |
22-
May -14 |
| US20140141
452 |
Method of Determining, Identifying or Isolating Cell-Penetrating Peptides | Phylogica Limited | 23-
May- 11 |
23-
May -12 |
22-
May -14 |
| US20140141
070 |
LIPOSOMES HAVING USEFUL N:P RATIO FOR DELIVERY OF RNA MOLECULES | Not Available | 6-Jul-
11 |
6-
Jul- 12 |
22-
May -14 |
| US20140141
033 |
CONJUGATED TLR7 AND/OR TLR8 AND TLR2 AGONISTS | CAYLA | 19-
Nov- 12 |
15-
Mar -13 |
22-
May -14 |
| US20140135
230 |
Lipoparticles Comprising Proteins, Methods Of Making, And Using The Same | Integral Molecular, Inc. | 30-
Jul-03 |
1-
Nov -13 |
15-
May -14 |
| US20140134
699 |
NOVEL STABILISATION METHOD FOR VIRUSES OR BACTERIA | Leukocare AG | 28-
Jun- 11 |
28-
Jun -12 |
15-
May -14 |
| US20140134
606 |
EXOSOME-MEDIATED DIAGNOSIS OF HEPATITIS VIRUS INFECTIONS AND DISEASES | MOREHOUSE SCHOOL OF MEDICINE | 6-
Oct- 08 |
21-
Jan -14 |
15-
May -14 |
| US20140134
206 |
INFLAMMATION AND IMMUNITY TREATMENTS | OCEAN SPRAY CRANBERRIES, INC. | 1-
Apr- 11 |
30-
Mar -12 |
15-
May -14 |
| US20140134
150 |
TECHNOLOGY FOR PREPARATION OF MACROMOLECULAR MICROSPHERES | Ansun Biopharma, Inc. | 24-
Jan- 06 |
6-
Jan -14 |
15-
May -14 |
| US20140128
324 |
METHODS AND COMPOSITIONS FOR THE TREATMENT OF CANCER OR OTHER DISEASES | CITY OF HOPE | 26-
Jan- 07 |
14-
Jan -14 |
8-
May -14 |
| US20140127
321 |
ANTIVIRAL RESIN MEMBER | NBC MESHTEC, INC. | 6-Jul-
11 |
6-
Jul- 12 |
8-
May -14 |
| US20140127
310 |
TREATMENT OF DISEASE WITH POLY-N-ACETYLGLUCOSAMINE NANOFIBERS | Marine Polymer Technologies, Inc. | 15-
Apr- 11 |
16-
Apr -12 |
8-
May -14 |
| US20140127
216 |
NOVEL EXPRESSION CASSETTE FOR EFFICIENT SURFACE DISPLAY OF ANTIGENIC PROTEINS | Temasek Life Sciences Laboratory Limited | 8-
Feb- 11 |
8-
Feb -12 |
8-
May -14 |
| US20140120
139 |
METHODS FOR INDUCING AN IMMUNE RESPONSE VIA BUCCAL AND/OR SUBLINGUAL ADMINISTRATION OF A VACCINE | Board of Regents, The University of Texas System | 26-
Jul-10 |
25-
Jan -13 |
1-
May -14 |
| US20140116
887 |
ELECTROCHEMISTRY AND ELECTROGENERATED CHEMILUMINESCENCE WITH A SINGLE FARADAIC ELECTRODE | Board of Regents of the University of Texas System | 3-
Jun- 05 |
28-
Oct -13 |
1-
May -14 |
| US20140113
855 |
Modified Release Formulations for Oprozomib | Not Available | 24-
Oct- 12 |
24-
Oct -13 |
24-
Apr- 14 |
| US20140107
133 |
3,5-DIAMINO-6-CHLORO-N-(N-(4-(4-(2-(HEXYL(2,3,4,5,6-
PENTAHYDROXYHEXYL)AMINO)ETHOXY)PHENYL)BUTYL)CARBAMIMI DOYL)PYRAZINE-2-CARBOXAMIDE |
Parion Sciences, Inc. | 27-
Jun- 11 |
18-
Dec -13 |
17-
Apr- 14 |
| US20140106
335 |
OLIGONUCLEOTIDE PROBE FOR THE DETECTION OF ADENOVIRUS | Qiagen Hamburg GMBH | 28-
Dec- 10 |
28-
Dec -10 |
17-
Apr- 14 |
| US20140105
928 |
Antiviral and antibacterial activity from medicinal mushrooms | Not Available | 6-
Jan- 04 |
20-
Dec -13 |
17-
Apr- 14 |
| US20140105
912 |
VISTA MODULATORS FOR DIAGNOSIS AND TREATMENT OF CANCER | THE TRUSTEES OF DARTMOUTH COLLEGE | 7-
Sep- 12 |
9-
Sep -13 |
17-
Apr- 14 |
| US20140099
696 |
Technology for the Preparation of Microparticles | Ansun Biopharma, Inc. | 24-
Jul-07 |
30-
Apr -13 |
10-
Apr- 14 |
| US20140099
323 |
METHODS FOR TREATMENT OF INFLAMMATORY DISEASES | Not Available | 8-
Apr- 09 |
30-
Oct -13 |
10-
Apr- 14 |
| US20140094
404 |
METHODS AND REAGENTS FOR EFFICIENT AND TARGETED DELIVERY OF THERAPEUTIC MOLECULES TO CXCR4 CELLS | Not Available | 13-
Jan- 11 |
13-
Jan -12 |
3-
Apr- 14 |
| US20140093
532 |
Immunogenic Compositions In Particulate Form And Methods For Producing The Same | MUCOSIS B.V. | 22-
Mar- 11 |
22-
Mar -12 |
3-
Apr- 14 |
| US20140087
954 |
QUANTITATIVE NUCLEASE PROTECTION ASSAY (QNPA) AND SEQUENCING (QNPS) IMPROVEMENTS | HTG Molecular Diagnostics, Inc. | 4-
May- 11 |
26-
Apr -12 |
27-
Mar -14 |
| US20140087
363 |
METHOD FOR GENERATING, STORING, TRANSPORTING, ELUTING AND DETECTING CLINICAL RELEVANT INFORMATION IN PLASMA USING FILTER PAPER | HVIDOVRE HOSPITAL | 9-
Dec- 10 |
9-
Dec -11 |
27-
Mar -14 |
| US20140086
928 |
Antigenic GM-CSF Peptides and Antibodies to GM-CSF | MORPHOTEK, INC. | 8-
Feb- 06 |
26-
Nov -13 |
27-
Mar -14 |
| US20140082
769 |
MOLECULES AND METHODS FOR INHIBITION AND DETECTION OF PROTEINS | Not Available | 11-
Mar- 11 |
12-
Mar -12 |
20-
Mar -14 |
| US20140080
828 |
IMIDAZOLYL AMIDE COMPOUNDS AND USES RELATED THERETO | CHILDREN’S HEATLHCARE OF ATLANTA, INC. | 31-
Mar- 11 |
28-
Mar -12 |
20-
Mar -14 |
| US20140080
171 |
OPTICAL CYTOMETRY | The Regents of the University of California | 6-
May- 09 |
25-
Nov -13 |
20-
Mar -14 |
| US20140080
121 |
PRIMATE T-LYMPHOTROPIC VIRUSES | Johns Hopkins University | 21-
Feb- 05 |
20-
Sep -13 |
20-
Mar -14 |
| US20140079
667 |
TETRAZOLONES AS INHIBITORS OF FATTY ACID SYNTHASE | Infinity Pharmaceuticals, Inc. | 5-
May- 10 |
23-
Aug -13 |
20-
Mar -14 |
| US20140073
683 |
MODIFIED SMALL INTERFERING RNA MOLECULES AND METHODS OF USE | Not Available | 26-
Jul-02 |
12-
Mar -13 |
13-
Mar -14 |
| US20140073
651 |
BENZAMIDE DERIVATIVES AS P2X7 RECEPTOR ANTAGONISTS | Actelion Pharmaceuticals Ltd. | 22-
Feb- 11 |
21-
Feb -12 |
13-
Mar -14 |
| US20140072
956 |
COMPOSITION, DEVICE AND ASSOCIATED METHOD | General Electric Company | 15-
Dec- 05 |
28-
Feb -07 |
13-
Mar -14 |
| US20140072
575 |
RSV-SPECIFIC BINDING MOLECULES AND MEANS FOR PRODUCING THEM | MedImmune Limited | 1-
Jun- 07 |
13-
Sep -13 |
13-
Mar -14 |
| US20140066
592 |
METHODS AND COMPOSITIONS FOR THE TREATMENT OF CANCER OR OTHER DISEASES | City of Hope | 26-
Jan- 07 |
11-
Oct -13 |
6-
Mar -14 |
| US20140057
926 |
SUBSTITUTED 4-PYRIDONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | BOEHRINGER INGELHEIM INTERNATIONAL GMBH | 23-
Aug- 12 |
20-
Aug -13 |
27-
Feb -14 |
| US20140057
920 |
SUBSTITUTED 4-PYRIDONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | BOEHRINGER INGELHEIM INTERNATIONAL GMBH | 23-
Aug- 12 |
20-
Aug -13 |
27-
Feb -14 |
| US20140057
916 |
SUBSTITUTED 4-PYRIDONES AND THEIR USE AS INHIBITORS OF NEUTROPHIL ELASTASE ACTIVITY | BOEHRINGER INGELHEIM INTERNATIONAL GMBH | 23-
Aug- 12 |
20-
Aug -13 |
27-
Feb -14 |
| US20140057
315 |
ANTIGEN PRESENTING CELL ASSAY | University of Pittsburgh – Of the Com monwealth System of Higher Education | 8-
Apr- 10 |
11-
Oct -13 |
27-
Feb -14 |
| US20140056
929 |
IMMUNOMODULATION BY CONTROLLING INTERFERON-GAMMA LEVELS WITH THE LONG NON-CODING RNA NeST | The Board of Trustees of the Leland Stanford Junior University | 23-
Aug- 12 |
23-
Aug -13 |
27-
Feb -14 |
| US20140051
148 |
Cells and Methodology to Generate Non-Segmented NegativeStrand RNA Viruses | Centre National De La Recherche Scientifique | 22-
Dec- 06 |
15-
Oct -13 |
20-
Feb -14 |
| US20140050
778 |
NUCLEIC ACID BINDING COMPOUNDS, METHODS OF MAKING, AND USE THEREOF | UNIVERSITY OF ROCHESTER | 28-
Dec- 10 |
28-
Dec -11 |
20-
Feb -14 |
| US20140050
763 |
MODIFIED VIRAL PARTICLES WITH IMMUNOGENIC PROPERTIES AND REDUCED LIPID CONTENT USEFUL FOR TREATING AND PREVENTING INFECTIOUS DISEASES | ELI LILLY & COMPANY | 29-
Jun- 00 |
30-
Oct -13 |
20-
Feb -14 |
| US20140050
753 |
POLYIONIC PAPILLOMA VIRUS-LIKE PARTICLE (VLP) VACCINES | THE JOHNS HOPKINS UNIVERSITY | 8-
Sep- 10 |
8-
Sep -11 |
20-
Feb -14 |
| US20140050
698 |
RECOMBINANT SUPER-COMPOUND INTERFERON AND USES THEREOF | Superlab Far East Limited | 28-
Feb- 01 |
5-
Sep -13 |
20-
Feb -14 |
| US20140045
919 |
Folate Conjugates | Alnylam Pharmaceuticals, Inc. | 4-
Dec- 07 |
8-
Jul- 13 |
13-
Feb -14 |
| US20140045
837 |
9-Substituted 8-Oxoadenine Compound | AstraZeneca Aktiebolag | 26-
Mar- 04 |
21-
Oct -13 |
13-
Feb -14 |
| US20140045
774 |
METHODS FOR TREATING VIRAL DISORDERS | TRUSTEES OF BOSTON UNIVERSITY | 24-
Sep- 09 |
11-
Jun -13 |
13-
Feb -14 |
| US20140045
743 |
COMPOSITIONS AND METHODS FOR CORONAVIRUS INHIBITION | Autoimmune Technologies, LLC | 4-
Nov- 03 |
8-
Aug -13 |
13-
Feb -14 |
| US20140044
641 |
POLYPEPTIDES AND POLYNUCLEOTIDES, AND USES THEREOF FOR TREATMENT OF IMMUNE RELATED DISORDERS AND CANCER | COMPUGEN LTD. | 15-
Apr- 11 |
16-
Apr -12 |
13-
Feb -14 |
| US20140038
978 |
HETEROCYCLIC COMPOUNDS AS CCR2B ANTAGONISTS | AstraZeneca AB | 24-
Dec- 04 |
13-
Sep -13 |
6-
Feb -14 |
| US20140038
174 |
Compositions and Methods for Detecting and Identifying Nucleic Acid Sequences in Biological Samples | LONGHORN VACCINES AND DIAGNOSTICS, LLC | 26-
Apr- 11 |
8-
Oct -13 |
6-
Feb -14 |
| US20140038
170 |
APPARATUS, SYSTEMS, AND METHODS FOR PERFORMING THERMAL MELT ANALYSES AND AMPLIFICATIONS | Not Available | 31-
Jul-12 |
31-
Jul- 13 |
6-
Feb -14 |
| US20140037
700 |
Composition and Method for Enhancing an Immune Response | MICO BIO, INC. | 20-
Apr- 11 |
19-
Apr -12 |
6-
Feb -14 |
| US20140031
418 |
Regimens and Compositions for AAV-Mediated Passive Immunization of Airborne Pathogens | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | 20-
Apr- 11 |
20-
Apr -12 |
30-
Jan- 14 |
| US20140031
371 |
POLY AROMATIC SODIUM CHANNEL BLOCKERS | PARION SCIENCES, Inc. | 26-
Feb- 08 |
1-
Oct -13 |
30-
Jan- 14 |
| US20140031
333 |
COMPOUNDS AND COMPOSITIONS AS C-KIT KINASE INHIBITORS | IRM LLC | 1-
Sep- 11 |
19-
Sep -13 |
30-
Jan- 14 |
| US20140031
276 |
COMPOUNDS AND METHODS FOR PREVENTING OR TREATING A VIRAL INFECTION | CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (C.N.R.S.) | 2-
Nov- 07 |
19-
Jun -13 |
30-
Jan- 14 |
| US20140030
740 |
Rapid Bioluminescence Detection System | The Secretary of State for Health | 7-
Jan- 09 |
2-
Aug -13 |
30-
Jan- 14 |
| US20140030
725 |
MUTANT PROTEASE BIOSENSORS WITH ENHANCED DETECTION CHARACTERISTICS | PROMEGA CORPORATION | 11-
May- 10 |
12-
Nov -12 |
30-
Jan- 14 |
| US20140030
703 |
Compositions and Methods for Detecting and Identifying Nucleic Acid Sequences in Biological Samples | LONGHORN VACCINES AND DIAGNOSTICS, LLC | 12-
Sep- 06 |
8-
Oct -13 |
30-
Jan- 14 |
| US20140030
364 |
ANTIVIRAL COMPOSITIONS COMPRISING ETHANOL EXTRACT OF TETRACERA SCANDENS AND USE THEREOF | Not Available | 24-
Jul-12 |
28-
Mar -13 |
30-
Jan- 14 |
| US20140030
295 |
Use of Si rt 1 Activators or Inhibitors to Modulate an Immune Response | THE J. DAVID GLADSTONE INSTITUTES | 7-
Feb- 08 |
17-
Dec -12 |
30-
Jan- 14 |
| US20140024
804 |
Crystalline Tripeptide Epoxy Ketone Protease Inhibitors | Onyx Therapeutics, Inc. | 20-
Mar- 09 |
24-
Sep -13 |
23-
Jan- 14 |
| US20140024
583 |
ORGANIC COMPOUNDS | Not Available | 5-
Jun- 06 |
15-
Jul- 13 |
23-
Jan- 14 |
| US20140023
645 |
ENGINEERED ANTIBODY CONSTANT DOMAIN MOLECULES | The United States of America, as represented by the Secretary, Dept. of Health & Human Services | 31-
Jan- 08 |
1-
Oct -13 |
23-
Jan- 14 |
| US20140023
592 |
SELF COUPLING RECOMBINANT ANTIBODY FUSION PROTEINS | Fraunhofer-Gesellschaft zur Forderung der Angewandten Forschung e.V. | 25-
Jul-07 |
30-
Sep -13 |
23-
Jan- 14 |
| US20140017
727 |
Cna-B DOMAIN ANTIGENS IN VACCINES AGAINST GRAM POSITIVE BACTERIA | Not Available | 12-
Jan- 09 |
7-
Jun -13 |
16-
Jan- 14 |
| US20140017
285 |
ADJUVANT NANOEMULSIONS WITH PHOSPHOLIPIDS | NOVARTIS AG | 24-
Mar- 11 |
23-
Mar -12 |
16-
Jan- 14 |
| US20140017
279 |
ADJUVANT NANOEMULSIONS WITH CRYSTALLISATION INHIBITORS | Novartis AG | 27-
Jan- 11 |
27-
Jan -12 |
16-
Jan- 14 |
| US20140011
982 |
Antibodies Against Influenza Virus and Methods of Use Thereof | BURNHAM INSTITUTE FOR MEDICAL RESEARCH | 6-
Dec- 07 |
12-
Aug -13 |
9-
Jan- 14 |
| US20140011
812 |
Methods of Treating Inflammation | Massachusetts Institute of Technology | 8-
Oct- 10 |
7-
Oct -11 |
9-
Jan- 14 |
| US20140011
777 |
SUBSTITUTED IMIDAZOQUINOLINES, IMIDAZOPYRIDINES, AND IMIDAZONAPHTHYRIDINES | 3M INNOVATIVE PROPERTIES COMPANY | 18-
Jun- 04 |
9-
Sep -13 |
9-
Jan- 14 |
| US20140011
187 |
Biological Specimen Collection and Transport System and Method of Use | Longhorn Vaccines and Diagnostics, LLC | 1-
Oct- 07 |
19-
Mar -13 |
9-
Jan- 14 |
| US20140010
837 |
Methods and Compositions for Preventing a Condition | Cyvax, Inc. | 9-
Aug- 10 |
11-
Sep -13 |
9-
Jan- 14 |
| US20140005
241 |
Suppression of Sars Replication by Sars Helicase Inhibitors | The Curators of the University of Missouri | 15-
Jun- 12 |
17-
Jun -13 |
2-
Jan- 14 |
| US20140005
102 |
Oligoadenylate Synthetase (OAS) | Kineta Two LLC | 23-
Nov- 05 |
1-
Jul- 13 |
2-
Jan- 14 |
| US20140005
101 |
Oligoadenylate Synthetase (OAS) | Kineta Two LLC | 23-
Nov- 05 |
14-
Jun -13 |
2-
Jan- 14 |
| US20140005
097 |
BIOACTIVE PEPTIDES AND METHODS OF USING SAME | Compugen Ltd. | 12-
Jul-07 |
21-
Jun -13 |
2-
Jan- 14 |
| US20140004
193 |
COMPOSITIONS AND METHODS FOR TREATING AND PREVENTING PORCINE REPRODUCTIVE AND RESPIRATORY SYNDROME | Not Available | 24-
Apr- 12 |
24-
Apr -13 |
2-
Jan- 14 |
| US20140004
146 |
METHOD FOR PRODUCING VIRUS-LIKE PARTICLE BY USING DROSOPHILA CELL AND APPLICATIONS THEREOF | INSTITUT PASTEUR OF SHANGHAI, CHINESE ACADEMY OF SCIENCES | 17-
Mar- 11 |
19-
Mar -12 |
2-
Jan- 14 |
| US20140004
143 |
ADENO-ASSOCIATED VIRUS (AAV) SEROTYPE 8 SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREFOR | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | 17-
Dec- 01 |
13-
Sep -13 |
2-
Jan- 14 |
| US20140004
128 |
ANTI-IL-18 ANTIBODIES AND THEIR USES | Medimmune Limited | 20-
Dec- 10 |
20-
Dec -11 |
2-
Jan- 14 |
| US20140004
123 |
MONOCLONAL ANTIBODY PRODUCTION BY EBV TRANSFORMATION OF B CELLS | Institute For Research in Biomedicine | 26-
Feb- 03 |
25-
Apr -13 |
2-
Jan- 14 |
| US20130345
414 |
TREATING CANCER WITH VIRAL NUCLEIC ACID | Not Available | 20-
Feb- 07 |
26-
Jul- 13 |
26-
Dec -13 |
| US20130345
284 |
siRNA Compositions and Methods for Treatment of HPV and Other Infections | Not Available | 29-
Jul-10 |
29-
Jan -13 |
26-
Dec -13 |
| US20130345
270 |
METHODS OF TREATING CANCER AND OTHER DISORDERS | Waake Forest University Health Sciences | 12-
Nov- 10 |
7-
Nov -11 |
26-
Dec -13 |
| US20130344
613 |
USE OF A FLUORESCENT MATERIAL TO DETECT FAILURE OR DETERIORATED PERFORMANCE OF A FLUOROMETER | GEN-PROBE INCORPORATED | 14-
Jun- 12 |
7-
Jun -13 |
26-
Dec -13 |
| US20130344
100 |
VIRUS LIKE PARTICLE PRODUCTION IN PLANTS | MEDICAGO INC. | 22-
Dec- 10 |
22-
Dec -11 |
26-
Dec -13 |
| US20130341
206 |
AMPEROMETRIC GAS SENSOR | STERIS CORPORATION | 25-
Jun- 12 |
26-
Feb -13 |
26-
Dec -13 |
| US20130338
224 |
DECONTAMINATING COMPOSITION HAVING SIMULTANEOUSLY BACTERICIDAL, FUNGICIDAL AND VIROCIDAL PROPERTIES, METHODS FOR OBTAINING AND USING SAID COMPOSITION | HIGHTECH BIO-ACTIVITIES HOLDING GMBH | 29-
Mar- 04 |
7-
Aug -13 |
19-
Dec -13 |
| US20130338
174 |
PURINE DERIVATIVES | ABBOTT PHILLIP | 17-
Dec- 10 |
14-
Dec -11 |
19-
Dec -13 |
| US20130338
130 |
METHYLSULFONYLMETHANE (MSM) FOR TREATMENT OF DRUG RESISTANT MICROORGANISMS | Biogenic Innovations, LLC | 30-
Oct- 09 |
19-
Aug -13 |
19-
Dec -13 |
| US20130336
996 |
NOVEL COMPOSITIONS OF TLR7 AND/OR TLR8 AGONISTS CONJUGATED TO LIPIDS | CAYLA | 15-
Jun- 12 |
15-
Mar -13 |
19-
Dec -13 |
| US20130336
929 |
METHODS AND CELLS FOR IDENTIFYING RIG-I PATHWAY REGULATORS | KINETA, INC. | 25-
Feb- 11 |
23-
Feb -12 |
19-
Dec -13 |
| US20130336
923 |
Carbonic Anhydrase IX (G250) Antibodies and Methods of Use Thereof | DANA-FARBER CANCER INSTITUTE, INC. | 2-
Dec- 05 |
8-
May -13 |
19-
Dec -13 |
| US20130331
313 |
METHODS AND COMPOSITIONS FOR TREATING VIRAL OR VIRALLY- INDUCED CONDITIONS | HEMAQUEST PHARMACEUTICALS INC | 11-
Mar- 10 |
7-
Jun -13 |
12-
Dec -13 |
| US20130330
840 |
FLOW CYTOMETRY ANALYSIS OF MATERIALS ADSORBED TO METAL SALTS | NOVARTIS AG | 14-
Dec- 10 |
14-
Dec -11 |
12-
Dec -13 |
| US20130330
335 |
BIOINFORMATIC PROCESSES FOR DETERMINATION OF PEPTIDE BINDING | IOGENETICS, LLC | 23-
Mar- 10 |
21-
Mar -11 |
12-
Dec -13 |
| US20130324
559 |
DENDRIMER LIKE AMINO AMIDES POSSESSING SODIUM CHANNEL BLOCKER ACTIVITY FOR THE TREATMENT OF DRY EYE AND OTHER
MUCOSAL DISEASES |
PARION SCIENCES, INC. | 29-
May- 12 |
29-
May -13 |
5-
Dec -13 |
| US20130323
835 |
Mammalian Genes Involved in Infection | Zirus, Inc. | 15-
Jul-09 |
15-
Jul- 10 |
5-
Dec -13 |
| US20130323
819 |
EXPRESSION OF ANTIBODY OR A FRAGMENT THEREOF IN LACTOBACILLUS | ALVAREZ MIGUEL ANGEL | 5-
Aug- 10 |
4-
Aug -11 |
5-
Dec -13 |
| US20130317
080 |
MODIFIED iRNA AGENTS | ALNYLAM PHARMACEUTICALS, INC. | 15-
Sep- 10 |
14-
Sep -11 |
28-
Nov -13 |
| US20130317
031 |
Nuclear Transport Modulators And Uses Thereof | Karyopharm Therapeutics Inc. | 9-
May- 12 |
9-
May -13 |
28-
Nov -13 |
| US20130315
952 |
ADMINISTRATION OF INTERFERON FOR PROPHYLAXIS AGAINST OR TREATMENT OF PATHOGENIC INFECTION | Defyrus, Inc. | 9-
Jun- 09 |
13-
Nov -12 |
28-
Nov -13 |
| US20130302
351 |
ANTAGONISM OF THE VIP SIGNALING PATHWAY | EMORY UNIVERSITY | 2-
Feb- 11 |
31-
Jan -12 |
14-
Nov -13 |
| US20130302
278 |
USE OF TLR AGONISTS AND/OR TYPE 1 INTERFERONS TO ALLEVIATE TOXICITY OF TNF-R AGONIST THERAPEUTIC REGIMENS | IMMURX INC | 15-
Jun- 07 |
16-
Apr -13 |
14-
Nov -13 |
| US20130295
129 |
IMMUNOSTIMULATORY COMPOSITIONS AND METHODS OF USE THEREOF | MASSACHUSETTS INSTITUTE OF TECHNOLOGY | 5-
Apr- 12 |
15-
Mar -13 |
7-
Nov -13 |
| US20130291
134 |
HUMANIZED TRANSGENIC MOUSE MODEL | BRUMEANU TEPDPR D | 24-
Sep- 10 |
22-
Aug -12 |
31-
Oct- 13 |
| US20130289
043 |
(4-TERT-BUTYLPIPERAZIN-2-YL)(PIPERAZIN-1-YL)METHANONE-N- CARBOXAMIDE DERIVATIVES | AstraZeneca AB | 15-
Dec- 08 |
27-
Jun -13 |
31-
Oct- 13 |
| US20130288
922 |
ARRAYED DETECTOR SYSTEM FOR MEASUREMENT OF INFLUENZA IMMUNE RESPONSE | UNIV ROCHESTER | 2-
May- 08 |
11-
Jul- 13 |
31-
Oct- 13 |
| US20130288
917 |
Rapid High Resolution, High Throughput RNA Structure, RNA-
Macromolecular Interaction, and RNA-Small Molecule Interaction Mapping |
The Board of Trustees of the Leland Stanford Junior University | 21-
Oct- 10 |
21-
Oct -11 |
31-
Oct- 13 |
| US20130287
832 |
SOLUBLE NEEDLE ARRAYS FOR DELIVERY OF INFLUENZA VACCINES | Novartis AG | 20-
Aug- 10 |
19-
Aug -11 |
31-
Oct- 13 |
| US20130287
811 |
Method for Producing Viral Vaccines | BAXTER HEALTHCARE SA | 28-
Aug- 07 |
26-
Jun -13 |
31-
Oct- 13 |
| US20130287
772 |
BIOMARKERS FOR THERANOSTICS | Caris Life Sciences Luxembourg Holdings | 1-
Mar- 10 |
1-
Mar -11 |
31-
Oct- 13 |
| US20130281
324 |
BI-FUNCTINAL COMPLEXES AND METHODS FOR MAKING AND USING SUCH COMPLEXES | Nuevolution A/S | 16-
Apr- 10 |
16-
Apr -11 |
24-
Oct- 13 |
| US20130280
806 |
MAMMALIAN GENES INVOLVED IN INFECTION | HODGE THOMAS | 17-
May- 10 |
17-
May -11 |
24-
Oct- 13 |
| US20130280
298 |
Immunogenic Affinity-Conjugated Antigen Systems Based on Papaya Mosaic Virus and Uses Thereof | LECLERC DENIS | 15-
Nov- 06 |
15-
Mar -13 |
24-
Oct- 13 |
| US20130274
465 |
ADSORPTION OF IMMUNOPOTENTIATORS TO INSOLUBLE METAL SALTS | IRM LLC | 1-
Sep- 10 |
1-
Sep -11 |
17-
Oct- 13 |
| US20130274
129 |
TAL-EFFECTOR ASSEMBLY PLATFORM, CUSTOMIZED SERVICES, KITS AND ASSAYS | ARNOLD MATTHIAS | 4-
Apr- 12 |
4-
Apr -13 |
17-
Oct- 13 |
| US20130273
540 |
METHOD FOR CHROMOGENIC DETECTION OF TWO OR MORE TARGET MOLECULES IN A SINGLE SAMPLE | BIENIARZ CHRISTOPHER A | 22-
Aug- 08 |
7-
Jun -13 |
17-
Oct- 13 |
| US20130273
277 |
POWDERED POUCH AND METHOD OF MAKING SAME | Monosol, LLC. | 16-
Apr- 12 |
14-
Mar -13 |
17-
Oct- 13 |
| US20130273
150 |
Dendritic Cell Marker and Uses Thereof | CAMINSCHI IRINA | 30-
Aug- 07 |
21-
Mar -13 |
17-
Oct- 13 |
| US20130273
120 |
METHODS AND COMPOSITIONS FOR INTRANASAL DELIVERY | HARUTA SHUNJI | 15-
Apr- 10 |
14-
Mar -13 |
17-
Oct- 13 |
| US20130273
113 |
IMMUNOGENIC APOPTOSIS INDUCING COMPOSITIONS AND METHODS OF USE THEREOF | The Regents of the University of Michigan | 12-
Apr- 12 |
15-
Mar -13 |
17-
Oct- 13 |
| US20130273
104 |
Adjuvanted Influenza Vaccines for Pediatric Use | GROTH NICOLA | 22-
Feb- 08 |
21-
Jun -13 |
17-
Oct- 13 |
| US20130273
080 |
SIALOADHESIN-RELATED COMPOSITIONS AND METHODS | DELPUTTE PETER | 11-
May- 06 |
15-
Mar -13 |
17-
Oct- 13 |
| US20130267
578 |
COMPOSITIONS AND METHODS FOR INHIBITING NADPH OXIDASE EXPRESSION | QUARK PHARMACEUTICALS, INC. | 15-
Jun- 07 |
6-
Jun -13 |
10-
Oct- 13 |
| US20130267
532 |
CYCLIC AMIDE COMPOUNDS AND THEIR USE IN THE TREATMENT OF DISEASE | HORI SEIJI | 19-
Nov- 10 |
18-
Nov -11 |
10-
Oct- 13 |
| US20130267
436 |
LUMINOPHORE-LABELED MOLECULES COUPLED WITH PARTICLES FOR MICROARRAY-BASED ASSAYS | CapitaBio Corporation | 27-
Oct- 10 |
27-
Oct -10 |
10-
Oct- 13 |
| US20130267
429 |
BIOLOGICAL SAMPLE TARGET CLASSIFICATION, DETECTION AND SELECTION METHODS, AND RELATED ARRAYS AND OLIGONUCLEOTIDE PROBES | GARDNER SHEA | 21-
Dec- 09 |
2-
May -13 |
10-
Oct- 13 |
| US20130266
645 |
DERIVATIVES OF STEROID BENZYLAMINES, HAVING AN ANTIPARASITIC ANTIBACTERIAL, ANTIMYCOTIC AND/OR ANTIVIRAL ACTION | Justus-Liebig-Universitat Giessen | 6-
Oct- 10 |
6-
Oct -11 |
10-
Oct- 13 |
| US20130266
612 |
VACCINE COMPOSITION | NITTO DENKO CORPORATION | 4-
Apr- 12 |
3-
Apr -13 |
10-
Oct- 13 |
| US20130261
196 |
Nucleic Acids For Multiplex Organism Detection and Methods Of Use And Making The Same | DIAMOND LISA | 11-
Jun- 10 |
10-
Jun -11 |
3-
Oct- 13 |
| US20130261
087 |
SULFONYL SEMICARBAZIDES, SEMICARBAZIDES AND UREAS, PHARMACEUTICAL COMPOSITIONS THEREOF, AND METHODS FOR TREATING HEMORRHAGIC FEVER VIRUSES, INCLUDING INFECTIONS ASSOCIATED WITH ARENA VIRUSES | SIGA TECHNOLOGIES INC. | 6-
Dec- 04 |
6-
Jul- 11 |
3-
Oct- 13 |
| US20130261
049 |
ALPHABODIES SPECIFICALLY BINDING TO CLASS-I VIRAL FUSION PROTEINS AND METHODS FOR PRODUCING THE SAME | COMPLIX SA | 6-
Jan- 11 |
2-
Dec -11 |
3-
Oct- 13 |
| US20130259
887 |
REOVIRUS VACCINES AND METHODS OF USE THEREFOR | BOEHME KARLW | 7-
Apr- 10 |
4-
Apr -11 |
3-
Oct- 13 |
| US20130259
871 |
Human Monoclonal Antibody with Specificity for Dengue Virus Serotype 1 E Protein and Uses Thereof | DSO NATIONAL LABORATORIES | 14-
Dec- 10 |
14-
Dec -11 |
3-
Oct- 13 |
| US20130259
829 |
CLIP INHIBITORS AND METHODS OF MODULATING IMMUNE FUNCTION | Viral Genetics, Inc. | 25-
Jul-08 |
6-
Jun -13 |
3-
Oct- 13 |
| US20130253
002 |
COMPOUNDS AND COMPOSITIONS AS TLR ACTIVITY MODULATORS | CORTEZ ALEX | 3-
Mar- 08 |
22-
Apr -13 |
26-
Sep -13 |
| US20130251
746 |
Methods and Compositions for Poxvirus A35R Protein | East Carolina University | 1-
Dec- 08 |
20-
Dec -12 |
26-
Sep -13 |
| US20130251
734 |
USE OF TAM RECEPTOR INHIBITORS AS ANTIMICROBIALS | The Salk Institute for Biological Studies | 9-
Nov- 07 |
8-
Mar -13 |
26-
Sep -13 |
| US20130251
590 |
Peracid/Peroxide Composition, Process for Accurately Making the Same, and Method for Use as an Evaporating Film Anti-Microbial Solution and as a Photosensitizer | BioMed Protect ,LLC | 11-
Jan- 05 |
21-
Sep -09 |
26-
Sep -13 |
| US20130244
980 |
BORON-CONTAINING SMALL MOLECULES | ANACOR PHARMACEUTICALS, INC. | 16-
Feb- 05 |
30-
Apr -13 |
19-
Sep -13 |
| US20130244
920 |
WATER SOLUBLE COMPOSITIONS INCORPORATING ENZYMES, AND METHOD OF MAKING SAME | LEE DAVID M | 16-
Mar- 12 |
16-
Mar -12 |
19-
Sep -13 |
| US20130243
841 |
CONCENTRATION OF VACCINE ANTIGENS WITH LYOPHILIZATION | Novartis AG | 1-
Jun- 10 |
1-
Jun -11 |
19-
Sep -13 |
| US20130243
756 |
Methods for Treating Diseases and HSV Using Antibodies to Aminophospholipids | BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM | 15-
Jul-02 |
19-
Feb -13 |
19-
Sep -13 |
| US20130243
727 |
TRIAZOLES AS INHIBITORS OF FATTY ACID SYNTHASE | INFINITY PHARMACEUTICALS, INC. | 5-
May- 10 |
29-
Apr -13 |
19-
Sep -13 |
| US20130237
507 |
Novel Amide Compounds | BURKAMP FRANK | 3-
Apr- 09 |
17-
Apr -13 |
12-
Sep -13 |
| US20130236
884 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF ORTHOPOXVIRUSES | IBIS BIOSCIENCES, INC. | 5-
Dec- 03 |
7-
May -13 |
12-
Sep -13 |
| US20130236
493 |
METHODS TO PRODUCE BUNYAVIRUS REPLICON PARTICLES | STICHTING DIENST LANDBOUWKUNDIG ONDERZOEK | 20-
Sep- 10 |
20-
Sep -11 |
12-
Sep -13 |
| US20130236
396 |
NANOPARTICLES PRODUCED FROM RECOMBINANT POLYMERS AND METHODS OF MAKING AND USING THE SAME | UNIVERSITY OF UTAH RESEARCH FOUNDATION | 3-
May- 10 |
3-
May -11 |
12-
Sep -13 |
| US20130231
302 |
ANTIMICROBIAL SOLUTIONS | BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM | 10-
Sep- 10 |
9-
Sep -11 |
5-
Sep -13 |
| US20130230
919 |
ANIMAL PROTEIN-FREE MEDIA FOR CULTIVATION OF CELLS | Baxter Healthcare S.A. | 29-
Oct- 04 |
16-
Apr -13 |
5-
Sep -13 |
| US20130230
856 |
CAPTURE OF TARGET DNA AND RNA BY PROBES COMPRISING INTERCALATOR MOLECULES | QUANTIBACT A/S | 27-
Oct- 10 |
27-
Oct -11 |
5-
Sep -13 |
| US20130230
851 |
Nanoreporters And Methods Of Manufacturing And Use Thereof | NANOSTRING TECHNOLOGIES, INC. | 23-
Dec- 05 |
11-
Mar -13 |
5-
Sep -13 |
| US20130230
578 |
LIPIDATED IMMUNE RESPONSE MODIFIER COMPOUND COMPOSITIONS, FORMULATIONS, AND METHODS | 3M INNOVATIVE PROPERTIES COMPANY | 17-
Aug- 10 |
16-
Aug -11 |
5-
Sep -13 |
| US20130230
529 |
NOVEL PARAMYXOVIRUS AND USES THEREOF | The University of Hong Kong | 20-
Jan- 12 |
22-
Jan -13 |
5-
Sep -13 |
| US20130225
655 |
Combinations of TGFBeta and COX-2 Inhibitors and Methods for Their Therapeutic Application | EVANS DAVID | 4-
May- 10 |
4-
May -11 |
29-
Aug -13 |
| US20130225
651 |
siRNA Compositions and Methods for Potently Inhibiting Viral Infection | The University of Hong Kong | 11-
Dec- 08 |
12-
Dec -11 |
29-
Aug -13 |
| US20130225
637 |
ISATIN DERIVATIVES, PHARMACEUTICAL COMPOSITIONS THEREOF, AND METHODS OF USE THEREOF | HORNE DAVID A | 24-
Feb- 12 |
25-
Feb -13 |
29-
Aug -13 |
| US20130225
555 |
Imidazoquinolines with Immuno-Modulating Properties | AstraZeneca AB | 8-
May- 07 |
14-
Mar -13 |
29-
Aug -13 |
| US20130224
245 |
CONCENTRATION OF VACCINE ANTIGENS WITHOUT LYOPHILIZATION | Novartis AG | 1-
Jun- 10 |
1-
Jun -11 |
29-
Aug -13 |
| US20130224
144 |
SEQUENTIAL ADMINISTRATION OF A REPLICATION DEFECTIVE ADENOVIRUS VECTOR IN VACCINATION PROTOCOLS | Etubics Corporation | 2-Jul-
07 |
18-
Sep -12 |
29-
Aug -13 |
| US20130217
584 |
MICROARRAY-BASED ASSAY INTEGRATED WITH PARTICLES FOR ANALYZING MOLECULAR INTERACTIONS | CapitalBio Corporation | 6-
Aug- 10 |
6-
Aug -10 |
22-
Aug -13 |
| US20130217
071 |
METHODS AND COMPOSITIONS FOR PERFORMING NUCLEIC ACID AMPLIFICATION REACTIONS | MONTESCLAROS LUZ | 30-
Dec- 11 |
21-
Dec -12 |
22-
Aug -13 |
| US20130217
012 |
C-CBL MUTATIONS AND USES THEREOF | The University of Chicago | 4-
Jun- 10 |
3-
Jun -11 |
22-
Aug -13 |
| US20130216
612 |
TARGETED INTRACELLULAR DELIVERY OF ANTIVIRAL AGENTS | BBB HOLDING B V | 23-
Mar- 07 |
2-
May -13 |
22-
Aug -13 |
| US20130216
581 |
Carbon Nanotube Compositions and Methods of Use Thereof | UNIV YALE | 19-
Mar- 08 |
15-
Mar -13 |
22-
Aug -13 |
| US20130216
573 |
INFLUENZA VIRUS REASSORTMENT | NOVARTIS AG | 21-
May- 10 |
20-
May -11 |
22-
Aug -13 |
| US20130216
566 |
Vectors expressing SARS immunogens, compositions containing such vectors or expression products thereof, methods and assays for making and using | ADAMS DANIEL | 20-
Jun- 03 |
21-
Jun -04 |
22-
Aug -13 |
| US20130210
894 |
APPLICATION OF HIGHLY CONSERVED DOMAIN SEQUENCES FROM VIRAL GENOME AS TEMPLATE TO DESIGN THERAPEUTIC SLIRNAS | CHEN LI | 20-
Sep- 10 |
19-
Sep -11 |
15-
Aug -13 |
| US20130210
820 |
Pharmaceutical Compounds | ASTEX THERAPEUTICS LIMITED | 11-
Apr- 08 |
30-
Jan -13 |
15-
Aug -13 |
| US20130210
770 |
PHARMACEUTICAL FORMULATIONS | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
12-
Apr -13 |
15-
Aug -13 |
| US20130209
550 |
Anti-Viral Azide Containing Compounds | LIFE TECHNOLOGIES CORPORATION | 28-
Jul-10 |
28-
Jul- 11 |
15-
Aug -13 |
| US20130209
510 |
METHODS FOR PREPARING SQUALENE | Novartis AG | 12-
May- 10 |
12-
May -11 |
15-
Aug -13 |
| US20130209
502 |
ALUMINA NANOPARTICLE BIOCONJUGATES AND METHODS OF STIMULATING AN IMMUNE RESPONSE USING SAID BIOCONJUGATES | HU HONG-MING | 27-
Aug- 10 |
26-
Aug -11 |
15-
Aug -13 |
| US20130209
406 |
RECOMBINANT RNA VIRUSES AND USES THEREOF | TENOEVER BENJAMIN R | 6-
Jun- 10 |
6-
Jun -11 |
15-
Aug -13 |
| US20130209
364 |
Anti-Viral Azide Containing Compounds | LIFE TECHNOLOGIES CORPORATION | 28-
Jul-10 |
28-
Jul- 11 |
15-
Aug -13 |
| US20130203
836 |
2′ AND 5′ MODIFIED MONOMERS AND OLIGONUCLEOTIDES | ALNYLAM PHARMACEUTICALS, INC. | 1-
Apr- 10 |
31-
Mar -11 |
8-
Aug -13 |
| US20130203
655 |
METHODS FOR PREVENTING OR TREATING VIRAL INFECTION | KIELIAN MARGARET | 12-
Feb- 10 |
9-
Feb -11 |
8-
Aug -13 |
| US20130203
151 |
PRODUCTION OF ALPHAVIRUS REPLICON PARTICLES IN PACKAGING CELLS | BALSITIS SCOTT | 26-
Apr- 10 |
26-
Apr -11 |
8-
Aug -13 |
| US20130202
684 |
PEGYLATED LIPOSOMES FOR DELIVERY OF IMMUNOGEN ENCODING RNA | Lichtstrasse | 31-
Aug- 10 |
31-
Aug -11 |
8-
Aug -13 |
| US20130202
609 |
GAS57 MUTANT ANTIGENS AND GAS57 ANTIBODIES | GRANDI GUIDO | 12-
Sep- 07 |
14-
Mar -13 |
8-
Aug -13 |
| US20130202
598 |
ACTIVATABLE TOXIN COMPLEXES COMPRISING A CLEAVABLE INHIBITORY PEPTIDE | RAMOT AT TEL-AVIV UNIVERSITY LTD. | 20-
Sep- 10 |
22-
Aug -11 |
8-
Aug -13 |
| US20130202
593 |
INTRACELLULAR IMMUNITY | MEDICAL RESEARCH COUNCIL | 23-
Jul-10 |
23-
Jan -13 |
8-
Aug -13 |
| US20130197
296 |
Removing Cells from an Organism | DAPPRICH JOHANNES | 13-
Jan- 12 |
14-
Jan -13 |
1-
Aug -13 |
| US20130196
903 |
Multimeric Inhibitors of Viral Fusion and Uses Thereof | JV BIO SRL | 11-
Aug- 10 |
11-
Aug -11 |
1-
Aug -13 |
| US20130196
380 |
IN VITRO PROCESS FOR THE PREPARATION OF ANTIBODIES OF THE IGG TYPE | JANSEN GABRIELE | 5-
Feb- 10 |
4-
Feb -11 |
1-
Aug -13 |
| US20130195
969 |
SMALL LIPOSOMES FOR DELIVERY OF IMMUNOGEN ENCODING RNA | NOVARTIS AG | 31-
Aug- 10 |
31-
Aug -11 |
1-
Aug -13 |
| US20130195
968 |
VIRION-LIKE DELIVERY PARTICLES FOR SELF-REPLICATING RNA MOLECULES | Novartis AG | 6-Jul-
10 |
6-
Jul- 11 |
1-
Aug -13 |
| US20130195
932 |
ARCHAEAL POLAR LIPID AGGREGATES FOR ADMINISTRATION TO ANIMALS | NATIONAL RESEARCH COUNCIL OF CANADA | 15-
Dec- 06 |
23-
Jan -13 |
1-
Aug -13 |
| US20130195
930 |
Inulin and Inulin Acetate Formulations | SOUTH DAKOTA SATE UNIVERSITY | 20-
Jan- 12 |
22-
Jan -13 |
1-
Aug -13 |
| US20130195
914 |
Expression Of Positive Sense Single Stranded RNA Virus And Uses Thereof | Viracine Therapeutics Corporation | 12-
Apr- 10 |
8-
Apr -11 |
1-
Aug -13 |
| US20130195
893 |
USE OF A PNEUMOCOCCAL P4 PEPTIDE FOR ENHANCING OPSONOPHAGOCYTOSIS IN RESPONSE TO A PATHOGEN | The Government of the United States of America as represented by the Secretary of the department of | 31-
Jul-08 |
27-
Mar -13 |
1-
Aug -13 |
| US20130195
792 |
METHOD OF TREATING INFLAMMATION | CYTOSORBENTS CORPORATION | 1-
Apr- 10 |
1-
Apr -11 |
1-
Aug -13 |
| US20130190
494 |
PURINE ANALOGS | THE REGENTS OF THE UNIVERSITY OF CALIFORNIA | 31-
May- 06 |
8-
Mar -13 |
25-
Jul- 13 |
| US20130190
381 |
INHIBITORS OF LONG AND VERY LONG CHAIN FATTY ACID METABOLISM AS BROAD SPECTRUM ANTI-VIRALS | THE TRUSTEES OF PRINCETON UNIVERSITY | 18-
Feb- 10 |
18-
Feb -11 |
25-
Jul- 13 |
| US20130190
206 |
Direct Clone Analysis and Selection Technology | Dublin City University | 13-
Jul-10 |
13-
Jul- 11 |
25-
Jul- 13 |
| US20130189
680 |
POLYMER STABILIZATION OF CHROMOGEN SOLUTIONS | VENTANA MEDICAL SYSTEMS, INC. | 23-
Jan- 12 |
18-
Jan -13 |
25-
Jul- 13 |
| US20130189
351 |
LIPIDS SUITABLE FOR LIPOSOMAL DELIVERY OF PROTEIN CODING RNA | NOVARTIS AG | 31-
Aug- 10 |
31-
Aug -11 |
25-
Jul- 13 |
| US20130189
311 |
ARRANGING INTERACTION AND BACK PRESSURE CHAMBERS FOR MICROFLUIDIZATION | Novartis AG | 3-
Dec- 09 |
3-
Dec -10 |
25-
Jul- 13 |
| US20130189
247 |
Multimeric Proteins Comprising Immunoglobulin Constant Domains | RESEARCH CORPORATION TECHNOLOGIES, INC. | 12-
Feb- 10 |
11-
Feb -11 |
25-
Jul- 13 |
| US20130184
170 |
OLIGONUCLEOTIDE AMPLIFICATION PRIMERS FOR TARGETING ONCOGENIC HPV | GENERA BIOSYSTEMS LIMITED | 5-
Apr- 07 |
4-
Jun -12 |
18-
Jul- 13 |
| US20130183
670 |
METHOD FOR PRODUCING NUCLEIC ACID PROBES | Ventana Medical Systems, Inc. | 1-
Sep- 06 |
11-
Mar -13 |
18-
Jul- 13 |
| US20130183
355 |
DELIVERY OF SELF-REPLICATING RNA USING BIODEGRADABLE POLYMER PARTICLES | NOVARTIS AG | 6-Jul-
10 |
7-
Jun -11 |
18-
Jul- 13 |
| US20130183
341 |
INFLUENZA VIRUS-LIKE PARTICLES (VLPS) COMPRISING HEMAGGLUTININ PRODUCED WITHIN A PLANT | MEDICAGO INC. | 13-
Jul-07 |
4-
Jan -13 |
18-
Jul- 13 |
| US20130183
321 |
GITR BINDING MOLECULES AND USES THEREFOR | GITR, INC. | 25-
Mar- 05 |
1-
Mar -13 |
18-
Jul- 13 |
| US20130178
512 |
CARBOHYDRATE CONJUGATES AS DELIVERY AGENTS FOR OLIGONUCLEOTIDES | ALNYLAM PHARMACEUTICALS, INC | 4-
Dec- 07 |
4-
Dec -12 |
11-
Jul- 13 |
| US20130178
482 |
3,5-DIAMINO-6-CHLORO-N-(N-(4-(4-(2-(HEXYL(2,3,4,5,6-
PENTAHYDROXYHEXYL)AMINO)ETHOXY)PHENYL)BUTYL) CARBAMIMIDOYL)PYRAZINE-2-CARBOXAMIDE |
Parion Sciences, Inc. | 27-
Jun- 11 |
26-
Jun -12 |
11-
Jul- 13 |
| US20130178
466 |
Treatment of Microbial Infections | BIOCOPEA LIMITED | 11-
Nov- 09 |
9-
Nov -10 |
11-
Jul- 13 |
| US20130178
448 |
Treatment of Respiratory Disorders | BANNISTER ROBIN MARK | 5-
Feb- 10 |
4-
Feb -11 |
11-
Jul- 13 |
| US20130178
383 |
VESICLE ISOLATION METHODS | KLASS MICHAEL | 12-
Nov- 08 |
29-
Aug -12 |
11-
Jul- 13 |
| US20130178
372 |
Methods And Computer Systems For Identifying Target-Specific Sequences For Use In Nanoreporters | NANOSTRING TECHNOLOGIES, INC. | 10-
Apr- 07 |
7-
Mar -13 |
11-
Jul- 13 |
| US20130177
640 |
DELIVERY OF RNA TO DIFFERENT CELL TYPES | NOVARTIS AG | 6-Jul-
10 |
7-
Jun -11 |
11-
Jul- 13 |
| US20130177
639 |
DELIVERY OF RNA TO TRIGGER MULTIPLE IMMUNE PATHWAYS | NOVARTIS AG | 6-Jul-
10 |
6-
Jul- 11 |
11-
Jul- 13 |
| US20130177
477 |
DECONTAMINANT DISPENSER SUITABLE FOR USE AS A PROJECTILE | STERIS INC. | 6-
Mar- 07 |
28-
Feb -13 |
11-
Jul- 13 |
| US20130172
327 |
SUBSTITUTED N- [1-CYANO-2- (PHENYL) ETHYL] -2-AZABICYCLO [2.2.1] HEPTANE-3-CARBOXAMIDE INHIBITORS OF CATHEPSIN C | BOEHRINGER INGELHEIM INTERNATIONAL GMBH | 19-
Sep- 11 |
14-
Sep -12 |
4-
Jul- 13 |
| US20130171
241 |
LIPOSOMES WITH LIPIDS HAVING AN ADVANTAGEOUS PKA-VALUE FOR RNA DELIVERY | NOVARTIS AG | 6-Jul-
10 |
6-
Jul- 11 |
4-
Jul- 13 |
| US20130171
129 |
CONSTRAINED IMMUNOGENIC COMPOSITIONS AND USES THEREFOR | COULIBALY FASSELI JOSEPH | 23-
Jun- 10 |
23-
Jun -11 |
4-
Jul- 13 |
| US20130165
455 |
TLR AGONISTS | The Regents of The University of California | 22-
Aug- 05 |
20-
Nov -12 |
27-
Jun- 13 |
| US20130165
417 |
METHODS AND COMPOSITIONS FOR TREATING ACE2-RELATED DISORDERS | UNIVERSITY OF FLORIDA RESEARCH FOUNDATION | 23-
Apr- 10 |
23-
Apr -11 |
27-
Jun- 13 |
| US20130157
964 |
Blockade Of Inflammatory Proteases With Cyclic Peptides | THE REGENTS OF THE UNIVERSITY OF CALIFORNIA | 2-
Jun- 11 |
1-
Jun -12 |
20-
Jun- 13 |
| US20130157
876 |
Systems and Methods for Detecting Antibiotic Resistance | TESSARAE, LLC | 21-
Aug- 10 |
21-
Feb -13 |
20-
Jun- 13 |
| US20130157
283 |
RAPID PATHOGEN DIAGNOSTIC DEVICE AND METHOD | PRESIDENT AND FELLOWS OF HARVARD COLLEGE | 19-
Jan- 10 |
19-
Jan -11 |
20-
Jun- 13 |
| US20130157
254 |
Method and apparatus for two-step surface-enhanced raman spectroscopy | Real-Time Analyzers, Inc. | 16-
Dec- 11 |
16-
Dec -11 |
20-
Jun- 13 |
| US20130156
807 |
CONJUGATES OF SYNTHETIC TLR AGONISTS AND USES THEREFOR | The Regents of The University of California | 7-
Feb- 07 |
8-
Jan -13 |
20-
Jun- 13 |
| US20130149
375 |
IMMUNISATION OF LARGE MAMMALS WITH LOW DOSES OF RNA | GEALL ANDREW | 6-Jul-
10 |
6-
Jul- 11 |
13-
Jun- 13 |
| US20130149
194 |
ORGANIC PEROXIDE COMPOUNDS FOR MICROORGANISM INACTIVATION | GREGERSEN JENS PETER | 6-
May- 10 |
6-
May -11 |
13-
Jun- 13 |
| US20130145
484 |
ANTIBODY PRODUCING NON-HUMAN MAMMALS | MERUS B.V. | 27-
Jun- 08 |
25-
Jan -13 |
6-
Jun- 13 |
| US20130143
810 |
Virally-Inactivated Growth Factors-Containing Platelet Lysate Depleted of PDGF and VEGF and Preparation Method Thereof | BURNOUF THIERRY | 25-
May- 10 |
26-
Nov -12 |
6-
Jun- 13 |
| US20130143
203 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF ADVENTITIOUS VIRUSES | IBIS BIOSCIENCES, INC. | 3-
Mar- 05 |
21-
May -12 |
6-
Jun- 13 |
| US20130142
833 |
CIRCULATION OF COMPONENTS DURING HOMOGENIZATION OF EMULSIONS | Novartis AG | 3-
Dec- 09 |
3-
Dec -10 |
6-
Jun- 13 |
| US20130142
826 |
RECOMBINANT INFLUENZA VIRUS-LIKE PARTICLES (VLPS) PRODUCED IN TRANSGENIC PLANTS EXPRESSING HEMAGGLUTININ | MEDICAGO, INC. | 21-
Jan- 08 |
23-
Jan -13 |
6-
Jun- 13 |
| US20130142
773 |
Mutations in OAS1 Genes | KINETA TWO, LLC | 4-
May- 05 |
14-
Nov -12 |
6-
Jun- 13 |
| US20130137
678 |
Antiviral Compounds and Uses Thereof | MOUNT SINAI SCHOOL OF MEDICINE | 28-
May- 10 |
31-
May -11 |
30-
May -13 |
| US20130137
172 |
HIGH DENSITY SELF-CONTAINED BIOLOGICAL ANALYSIS | BioFire Diagnostics, Inc. f/k/a Idaho Technology, Inc. | 15-
May- 09 |
28-
Jan -13 |
30-
May -13 |
| US20130137
140 |
INCREASED PROTEIN EXPRESSION THROUGH INCREASED MEMBRANE FORMATION | UNIVERSITEIT GENT | 17-
Jun- 10 |
15-
Jun -11 |
30-
May -13 |
| US20130136
768 |
Recombinant HCMV and RHCMV vectors and uses thereof | Oregon Health & Science University | 14-
May- 10 |
14-
Nov -12 |
30-
May -13 |
| US20130130
262 |
SAMPLE-TO-ANSWER MICROFLUIDIC CARTRIDGE | BATTRELL C FREDERICK | 29-
Jan- 10 |
28-
Jan -11 |
23-
May -13 |
| US20130129
805 |
COMPOSITION WITH STERILIZING ACTIVITY AGAINST BACTERIA, FUNGUS AND VIRUSES, APPLICATION THEREOF AND METHOD FOR PREPARATION THEREOF | GP&E | 18-
Nov- 11 |
18-
Nov -11 |
23-
May -13 |
| US20130129
786 |
HYDROPHILIC FILTRATION DURING MANUFACTURE OF VACCINE ADJUVANTS | Novartis AG | 3-
Dec- 09 |
3-
Dec -10 |
23-
May -13 |
| US20130129
782 |
DECREASING POTENTIAL IATROGENIC RISKS ASSOCIATED WITH INFLUENZA VACCINES | NOVARTIS AG | 9-
Sep- 04 |
11-
Jan -13 |
23-
May -13 |
| US20130129
781 |
METHODS AND COMPOSITIONS FOR INTRANASAL DELIVERY | HARUTA SHUNJI | 15-
Apr- 10 |
15-
Apr -11 |
23-
May -13 |
| US20130129
702 |
MATERIALS AND METHODS FOR PREVENTION AND TREATMENT OF RNA VIRAL DISEASES | UNIVERSITY OF SOUTH FLORIDA | 30-
Apr- 02 |
17-
Sep -12 |
23-
May -13 |
| US20130122
581 |
TAL EFFECTOR-MEDIATED DNA MODIFICATION | IOWA STATE UNIVERSITY RESEARCH FOUNDATION, INC. | 10-
Dec- 09 |
10-
Jan -13 |
16-
May -13 |
| US20130122
516 |
DETECTING TARGETS USING MASS TAGS AND MASS SPECTROMETRY | BIENIARZ CHRISTOPHER | 2-Jul-
10 |
1-
Jul- 11 |
16-
May -13 |
| US20130122
487 |
DECREASING POTENTIAL IATROGENIC RISKS ASSOCIATED WITH INFLUENZA VACCINES | Novartis AG | 9-
Sep- 04 |
10-
Jan -13 |
16-
May -13 |
| US20130122
043 |
MODIFIED POLYPEPTIDES AND PROTEINS AND USES THEREOF | WHITEHEAD INSTITUTE FOR BIOMEDICAL RESEARCH | 20-
Apr- 10 |
20-
Apr -11 |
16-
May -13 |
| US20130122
038 |
HETEROLOGOUS PRIME-BOOST IMMUNIZATION USING MEASLES VIRUS-BASED VACCINES | Crucell Holland B.V. | 14-
Nov- 11 |
13-
Nov -12 |
16-
May -13 |
| US20130122
031 |
HMGB1-DERIVED PEPTIDES ENHANCE IMMUNE RESPONSE TO ANTIGENS | MESSMER DAVORKA | 27-
Jul-10 |
27-
Jul- 11 |
16-
May -13 |
| US20130115
593 |
SUBSTRATES FOR CHROMOGENIC DETECTION AND METHODS OF USE IN DETECTION ASSAYS AND KITS | Ventana Medical Systems, Inc. | 16-
Aug- 10 |
12-
Aug -11 |
9-
May -13 |
| US20130115
244 |
Immunogenic Substances Comprising a Polyinosinic Acid – Polycytidilic Acid Based Adjuvant | Yisheng Biopharma (Singapore) PTE. LTD. | 13-
Jan- 06 |
1-
Oct -12 |
9-
May -13 |
| US20130115
228 |
IMMUNOSTIMULATORY COMBINATIONS | TRUSTEES OF DARTMOUTH COLLEGE | 30-
Dec- 02 |
22-
Oct -12 |
9-
May -13 |
| US20130115
215 |
DOMAIN INSERTION IMMUNOGLOBULIN | ZHOU HONGXING | 14-
Jul-10 |
14-
Jul- 11 |
9-
May -13 |
| US20130102
652 |
METHODS AND COMPOSITIONS RELATED TO MODIFIED ADENOSINES FOR CONTROLLING OFF-TARGET EFFECTS IN RNA INTERFERENCE | UNIVERSITY OF UTAH RESEARCH FOUNDATION | 23-
Mar- 10 |
23-
Mar -11 |
25-
Apr- 13 |
| US20130102
560 |
NUTRITIONAL COMPOSITION COMPRISING IMMUNOGLOBULINS AND OLIGOSACCHARIDES | N.V. NUTRICIA | 24-
Aug- 04 |
14-
Sep -12 |
25-
Apr- 13 |
| US20130102
524 |
Compounds And Their Use | Novabiotics Limited | 31-
Mar- 10 |
30-
Mar -11 |
25-
Apr- 13 |
| US20130101
629 |
HOMOGENOUS SUSPENSION OF IMMUNOPOTENTIATING COMPOUNDS AND USES THEREOF | NOVARTIS AG | 15-
Dec- 09 |
15-
Dec -10 |
25-
Apr- 13 |
| US20130101
622 |
IMIDAZOQUINOXALINE COMPOUNDS AS IMMUNOMODULATORS | Novartis AG | 23-
Mar- 06 |
7-
May -12 |
25-
Apr- 13 |
| US20130101
609 |
IRRADIATED BIODEGRADABLE POLYMER MICROPARTICLES | NOVARTIS AG | 24-
Jan- 10 |
24-
Jan -11 |
25-
Apr- 13 |
| US20130096
103 |
IMIDAZOQUINOLINE COMPOUNDS | NOVARTIS VACCINES & DIAGNOSTICS, INC. | 14-
Sep- 04 |
19-
Jun -12 |
18-
Apr- 13 |
| US20130096
073 |
Casein Derived Peptides And Uses Thereof | SIDELMAN ZVI | 1-
Mar- 00 |
5-
Sep -12 |
18-
Apr- 13 |
| US20130096
056 |
AMINOTHIAZOLE DERIVATIVES AS HUMAN STEAROYL-COA DESATURASE INHIBITORS | XENON PHARMACEUTICALS INC. | 3-
Jun- 05 |
14-
Sep -12 |
18-
Apr- 13 |
| US20130096
020 |
GENERATION OF BINDING MOLECULES | MERUS BIOPHARMACEUTICALS B.V. | 26-
Sep- 11 |
26-
Sep -12 |
18-
Apr- 13 |
| US20130095
552 |
NOROVIRUS AND SAPOVIRUS ANTIGENS | Novartis Vaccines and Diagnostics, Inc. | 22-
Nov- 05 |
27-
Nov -12 |
18-
Apr- 13 |
| US20130095
135 |
PROCESS FOR REMOVING ADVENTITIOUS AGENTS DURING THE PRODUCTION OF A VIRUS IN CELL CULTURE | GlaxoSmithKline Biologicals S.A. | 8-Jul-
10 |
6-
Jul- 11 |
18-
Apr- 13 |
| US20130091
611 |
Anti-viral Formulations Nanomaterials and Nanoparticles | LAMBKIN-WILLIAMS ROBERT | 16-
Feb- 06 |
30-
Nov -12 |
18-
Apr- 13 |
| US20130087
700 |
DIRECT IMPACT IONIZATION (DII) MASS SPECTROMETRY | Services, National Institutes of Health | 11-
Oct- 11 |
11-
Oct -11 |
11-
Apr- 13 |
| US20130085
095 |
PROTEIN COMPLEMENTATION REGULATORS | Trustees of Boston University | 29-
Sep- 11 |
28-
Sep -12 |
4-
Apr- 13 |
| US20130084
303 |
PEPTIDES SHARED AMONG LETHAL CANCERS AND THERAPEUTIC COMPOSITIONS COMPRISING SAID PEPTIDES | BOGOCH ELENORE S | 7-
Aug- 09 |
19-
Jul- 12 |
4-
Apr- 13 |
| US20130071
428 |
PEPTIDES, CONJUGATES AND METHOD FOR INCREASING IMMUNOGENICITY OF A VACCINE | ACADEMISCH ZIEKENHUIS LEIDEN H.O.D.N. LUMC | 15-
Mar- 10 |
15-
Mar -11 |
21-
Mar -13 |
| US20130059
846 |
COMPOUNDS AND COMPOSITIONS AS C-KIT KINASE INHIBITORS | IRM LLC | 1-
Sep- 11 |
27-
Aug -12 |
7-
Mar -13 |
| US20130059
832 |
COMPOUNDS AND COMPOSITIONS AS C-KIT KINASE INHIBITORS | IRM LLC | 1-
Sep- 11 |
29-
Aug -12 |
7-
Mar -13 |
| US20130059
803 |
BORON-CONTAINING SMALL MOLECULES | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
7-
Sep -12 |
7-
Mar -13 |
| US20130059
802 |
BORON-CONTAINING SMALL MOLECULES | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
7-
Sep -12 |
7-
Mar -13 |
| US20130059
776 |
Antiviral Cell-Penetrating Peptides | CURRELI FRANCESCA | 6-
May- 08 |
31-
Oct -12 |
7-
Mar -13 |
| US20130058
945 |
Antigenic GM-CSF Peptides and Antibodies to GM-CSF | CHAO QIMIN | 8-
Feb- 06 |
24-
Oct -12 |
7-
Mar -13 |
| US20110070
575 |
Immunomodulatory Compositions, Combinations and Methods | Coley Pharmaceutical Group, Inc. | 8-
Dec- 04 |
8-
Dec -05 |
24-
Mar -11 |
| US20110067
121 |
Transgenic mice having a human major histocompatibility complex (MHC) phenotype, experimental uses and applications | INSTITUT PASTEUR | 30-
Jul-03 |
22-
Dec -09 |
17-
Mar -11 |
| US20110065
111 |
Compositions For Use In Genotyping Of Klebsiella Pneumoniae | Ibis Biosciences, Inc. | 31-
Aug- 09 |
27-
Aug -10 |
17-
Mar -11 |
| US20110065
091 |
Coronavirus, Nucleic Acid, Protein, and Methods for the Generation of Vaccine, Medicaments and Diagnostics | VAN DER HOEK CORNELIA MARIA | 18-
Aug- 03 |
26-
Jul- 10 |
17-
Mar -11 |
| US20110065
089 |
NOVEL STRAIN OF SARS-ASSOCIATED CORONAVIRUS AND APPLICATIONS THEREOF | CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE | 2-
Dec- 03 |
6-
Apr -10 |
17-
Mar -11 |
| US20110064
772 |
VIRAL ADJUVANTS | University of North Carolina at Chapel Hill | 9-Jul-
04 |
24-
Nov -10 |
17-
Mar -11 |
| US20110053
986 |
RESPIRATORY DISEASE TREATMENT | FINCH HARRY | 7-
Aug- 08 |
4-
Aug -10 |
3-
Mar -11 |
| US20110053
909 |
Compounds – 801 | ALCARAZ LILIAN | 31-
Jul-09 |
30-
Jul- 10 |
3-
Mar -11 |
| US20110053
894 |
Broad Spectrum Antiviral and Methods of Use | LOVELACE RESPIRATORY RESEARCH INSTITUTE | 17-
Apr- 06 |
17-
Apr -07 |
3-
Mar -11 |
| US20110053
893 |
COMPOUNDS AND COMPOSITIONS AS TLR ACTIVITY MODULATORS | IRM LLC | 2-
Sep- 09 |
1-
Sep -10 |
3-
Mar -11 |
| US20110053
831 |
COMPOSITIONS AND USES THEREOF FOR THE TREATMENT OF ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) AND CLINICAL DISORDERS ASSOCIATED WITH THEREWITH | Phylogica Limited | 20-
Jun- 07 |
20-
Jun -08 |
3-
Mar -11 |
| US20110053
169 |
METHOD FOR CONTINUOUS MODE PROCESSING OF THE CONTENTS OF MULTIPLE REACTION RECEPTACLES IN A REAL-TIME AMPLIFICATION ASSAY | GEN-PROBE INCORPORATED | 10-
Mar- 05 |
4-
Nov -10 |
3-
Mar -11 |
| US20110052
716 |
Adjuvant Comprising Aluminum, Oligonucleotide and Polycation | O’HAGAN DEREK | 27-
Aug- 09 |
27-
Aug -10 |
3-
Mar -11 |
| US20110052
558 |
MATERIALS AND METHODS FOR PREVENTION AND TREATMENT OF RNA VIRAL DISEASES | UNIVERSITY OF SOUTH FLORIDA | 30-
Apr- 02 |
15-
Dec -09 |
3-
Mar -11 |
| US20110046
155 |
HYDROBENZAMIDE DERIVATIVES AS INHIBITORS OF HSP90 | FREDERICKSON MARTYN | 12-
Oct- 06 |
12-
Oct -07 |
24-
Feb -11 |
| US20110046
134 |
NOVEL PIPERAZINE DERIVATIVES AS INHIBITORS OF STEAROYL- CoA DESATURASE | BISCHOFF ALEXANDER | 21-
Jun- 07 |
29-
Oct -10 |
24-
Feb -11 |
| US20110046
001 |
Multiplex Assay for Respiratory Viruses | AUTOGENOMICS, INC. | 20-
Nov- 07 |
20-
Nov -08 |
24-
Feb -11 |
| US20110045
456 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF ADVENTITIOUS CONTAMINANT VIRUSES | IBIS BIOSCIENCES, INC. | 14-
Jun- 07 |
12-
Jun -08 |
24-
Feb -11 |
| US20110045
024 |
RNA VIRUS VACCINES AND METHODS | DITTMER DIRK P | 14-
Dec- 05 |
2-
Aug -10 |
24-
Feb -11 |
| US20110045
022 |
VACCINES INCLUDING ANTIGEN FROM FOUR STRAINS OF INFLUENZA VIRUS | TSAI THEODORE | 6-
Dec- 06 |
5-
Dec -07 |
24-
Feb -11 |
| US20110039
884 |
Imidazopyridinones | Pfizer Limited | 3-
Aug- 07 |
28-
Oct -10 |
17-
Feb -11 |
| US20110038
935 |
ANTIBODIES AGAINST INFLUENZA VIRUS AND METHODS OF USE THEREOF | LIDDINGTON ROBERT C | 6-
Dec- 07 |
8-
Dec -08 |
17-
Feb -11 |
| US20110038
900 |
NANOPARTICLES FOR USE IN PHARMACEUTICAL COMPOSITIONS | NOVARTIS AG | 28-
Apr- 08 |
28-
Apr -09 |
17-
Feb -11 |
| US20110038
884 |
IMMUNOPOTENTIATING AGENT COMPRISING EP1 AGONIST | NATIONAL UNIVERSITY CO., HAMAMATSU UNIVER. SCHOOL OF MEDICINE | 28-
Apr- 08 |
27-
Apr -09 |
17-
Feb -11 |
| US20110038
879 |
IMMUNOGENIC AND THERAPEUTIC COMPOSITIONS FOR STREPTOCOCCUS PYOGENES | NOVARTIS AG | 30-
Oct- 06 |
30-
Oct -07 |
17-
Feb -11 |
| US20110038
852 |
ANTIVIRALS THAT TARGET TRANSPORTERS, CARRIERS, AND ION CHANNELS | 3-V BIOSCIENCES, INC. | 10-
Jun- 09 |
9-
Jun -10 |
17-
Feb -11 |
| US20110038
849 |
INHIBITORY POLYNUCLEOTIDE COMPOSITIONS AND METHODS FOR TREATING CANCER | Intradigm Corporation | 21-
Dec- 06 |
21-
Dec -06 |
17-
Feb -11 |
| US20110034
438 |
STRUCTURAL MIMETICS OF PROLINE-RICH PEPTIDES AND THE PHARMACEUTICAL USE THEREOF | FORSCHUNGSVERBUND BERLIN E.V. | 25-
Sep- 06 |
25-
Sep -07 |
10-
Feb -11 |
| US20110033
498 |
METHODS OF PREVENTING AND TREATING VIRAL INFECTIONS BY INHIBITING THE DelSGYLATION ACTIVITY OF OTU DOMAIN- CONTAINING VIRAL PROTEINS | FRIAS-STAHELI NATALIA | 5-
Apr- 07 |
7-
Apr -08 |
10-
Feb -11 |
| US20110033
485 |
ANALOGUES OF GLYCOLIPIDS USEFUL AS IMMUNOADJUVANTS | PANZA LUIGI | 12-
Oct- 07 |
10-
Oct -08 |
10-
Feb -11 |
| US20110033
454 |
Methods For Treating Diseases Using Antibodies to Aminophospolipids | Board of Regents, The University of Texas System | 15-
Jul-02 |
26-
Aug -10 |
10-
Feb -11 |
| US20110028
945 |
Device including altered microorganisms, and methods and systems of use | Searete LLC, | 14-
Dec- 05 |
28-
May -10 |
3-
Feb -11 |
| US20110028
715 |
NOVEL ADENINE COMPOUND | AstraZeneca Aktiebolag | 20-
Mar- 07 |
14-
Oct -10 |
3-
Feb -11 |
| US20110028
531 |
NOVEL SIRNA COMPOUNDS FOR INHIBITING RTP801 | FEINSTEIN ELENA | 20-
Mar- 08 |
17-
Mar -09 |
3-
Feb -11 |
| US20110028
334 |
CAPTURE PRIMERS AND CAPTURE SEQUENCE LINKED SOLID SUPPORTS FOR MOLECULAR DIAGNOSTIC TESTS | IBIS BIOSCIENCES, INC. | 31-
Jul-09 |
30-
Jul- 10 |
3-
Feb -11 |
| US20110027
315 |
PROTEIN CAGES AND THEIR USES | DOUGLAS TREVOR | 7-
Sep- 07 |
5-
Sep -08 |
3-
Feb -11 |
| US20110027
314 |
Influenza Vaccines Containing Hemagglutinin and Matrix Proteins | NOVARTIS VACCINES AND DIAGNOSTICS GMBH & CO. KG | 27-
Jan- 06 |
26-
Jan -07 |
3-
Feb -11 |
| US20110027
306 |
TC-83-Derived Alphavirus Vectors, Particles and Methods | AlphaVax, Inc. | 18-
May- 04 |
6-
Jul- 10 |
3-
Feb -11 |
| US20110027
267 |
Fusion Proteins of Mannose Binding Lectins for Treatment of Disease | ANAPHORE, INC. | 9-
Nov- 07 |
10-
Nov -08 |
3-
Feb -11 |
| US20110027
257 |
CLOTTABLE CONCENTRATE OF PLATELET GROWTH FACTORS AND PREPARATION METHOD THEREOF | GWO REI BIOMEDICAL TECHNOLOGY CORPORATION | 7-
Jan- 08 |
7-
Jan -09 |
3-
Feb -11 |
| US20110027
228 |
USES OF INTERFERONS WITH ALTERED SPATIAL STRUCTURE | WEI GUANGWEN | 28-
Aug- 03 |
15-
Oct -10 |
3-
Feb -11 |
| US20110027
181 |
Device including altered microorganisms, and methods and systems of use | Searete LLC | 14-
Dec- 05 |
28-
May -10 |
3-
Feb -11 |
| US20110021
861 |
HAZARDOUS SUBSTANCE-REMOVING MATERIAL | KOSUGI TAKUJI | 25-
Mar- 08 |
23-
Mar -09 |
27-
Jan- 11 |
| US20110021
606 |
RNAi Modulation of RSV, PIV and Other Respiratory Viruses and Uses Thereof | SOUTH ALABAMA MEDICAL SCIENCE FOUNDATION | 22-
Oct- 04 |
23-
Jul- 10 |
27-
Jan- 11 |
| US20110021
554 |
IMMUNE RESPONSE MODIFIER FORMULATIONS AND METHODS | GUY CYNTHIA A | 30-
Dec- 04 |
29-
Sep -10 |
27-
Jan- 11 |
| US20110021
415 |
ANTIMICROBIAL PEPTIDES | NOVABIOTICS LIMITED | 18-
Aug- 04 |
29-
Sep -10 |
27-
Jan- 11 |
| US20110020
897 |
RNA-DEPENDENT DNA POLYMERASE FROM GEOBACILLUS STEAROTHERMOPHILUS | LAMPSON BERT C | 10-
Mar- 04 |
1-
Mar -10 |
27-
Jan- 11 |
| US20110020
315 |
TREATMENT OF INFLAMMATORY ILLNESSES WITH ACE2 | LOIBNER HANS | 18-
Dec- 07 |
18-
Dec -08 |
27-
Jan- 11 |
| US20110009
414 |
HETEROCYCLIC DERIVATIVES AND THEIR USE AS THERAPEUTIC AGENTS | Xenon Pharmaceuticals Inc. | 20-
Sep- 04 |
6-
May -09 |
13-
Jan- 11 |
| US20110008
905 |
SYSTEM FOR DETECTING POLYNUCLEOTIDES | INVESTIGEN | 20-
May- 03 |
9-
Jun -10 |
13-
Jan- 11 |
| US20110008
838 |
CHIMERIC VARICELLA ZOSTER VIRUS VIRUS-LIKE PARTICLES | PUSHKO PETER | 19-
Jul-07 |
21-
Jul- 08 |
13-
Jan- 11 |
| US20110008
395 |
MIXED MICELLES INCLUDING AMPHIPATHIC CONJUGATES OF RNA AGENTS, AND USES THEREOF | LEVCHENKO TATYANA S | 6-Jul-
07 |
7-
Jul- 08 |
13-
Jan- 11 |
| US20110008
384 |
Antiviral activity from medicinal mushrooms | STAMETS PAUL EDWARD | 6-
Jan- 04 |
27-
Mar -07 |
13-
Jan- 11 |
| US20110008
283 |
INTERFERON FUSION PROTEINS | ARTYMIUK PETER | 8-
Aug- 07 |
5-
Aug -08 |
13-
Jan- 11 |
| US20110008
280 |
METHODS FOR TREATING VIRAL INFECTION USING IL-28 AND IL- 29 CYSTEINE MUTANTS | ZymoGenetics, Inc. | 2-
Apr- 04 |
20-
May -10 |
13-
Jan- 11 |
| US20110003
890 |
USE OF ANGIOGENESIS ANTAGONISTS IN CONDITIONS OF ABNORMAL VENOUS PROLIFERATION | KENNEDY THOMAS P | 8-
Nov- 07 |
10-
Nov -08 |
6-
Jan- 11 |
| US20110003
315 |
CHIMERIC PCSK9 PROTEINS, CELLS COMPRISING SAME, AND ASSAYS USING SAME | INSTITUT DE RECHERCHES CLINIQUES DE MONTREAL | 8-
May- 06 |
8-
May -07 |
6-
Jan- 11 |
| US20110002
958 |
Alphavirus Vectors for Respiratory Pathogen Vaccines | NOVARTIS VACCINES AND DIAGNOSTICS, INC. | 21-
May- 04 |
1-
Jun -10 |
6-
Jan- 11 |
| US20110002
946 |
Immunostimulatory Combinations | AHONEN CORY L | 30-
Dec- 02 |
29-
Jul- 10 |
6-
Jan- 11 |
| US20110002
888 |
Albumin Fusion Proteins | Human Gemone Sciences, Inc. | 21-
Dec- 01 |
3-
Jun -10 |
6-
Jan- 11 |
| US20110000
480 |
ADMINISTRATION OF INTERFERON FOR PROPHYLAXIS AGAINST OR TREATMENT OF PATHOGENIC INFECTION | ENNIS JANE E | 9-
Jun- 09 |
9-
Jun -10 |
6-
Jan- 11 |
| US20100330
592 |
METHOD FOR DETECTING TRUNCATED MOLECULES | KEY MARC E | 29-
Jan- 08 |
29-
Jan -09 |
30-
Dec -10 |
| US20100330
190 |
IMMUNOGENIC COMPOSITIONS AND METHODS OF USE THEREOF | BOZIA JADRANKA | 17-
Dec- 07 |
17-
Dec -08 |
30-
Dec -10 |
| US20100330
141 |
PRODUCT FOR ABSORPTION PURPOSES | HJERTEN MARIE-CHRISTINE | 13-
Jun- 03 |
13-
Sep -10 |
30-
Dec -10 |
| US20100330
140 |
DEVICES AND METHODS FOR DECREASING HUMAN PATHOGEN TRANSMISSION | FILLIGENT LIMITED | 26-
Jun- 07 |
25-
Jun -08 |
30-
Dec -10 |
| US20100330
122 |
VARICELLA ZOSTER VIRUS VIRUS-LIKE PARTICLES (VLPs) AND ANTIGENS | PUSHKO PETER | 19-
Jul-07 |
21-
Jul- 08 |
30-
Dec -10 |
| US20100330
114 |
Use of SIRT1 Activators or Inhibitors to Modulate an Immune Response | KWON HYE-SOOK | 7-
Feb- 08 |
16-
Jul- 10 |
30-
Dec -10 |
| US20100330
075 |
MONOCLONAL HUMAN TUMOR-SPECIFIC ANTIBODY | ABELA IRENE | 13-
Mar- 07 |
13-
Mar -08 |
30-
Dec -10 |
| US20100329
971 |
NOVEL HYDROXY RADICAL GENERATION METHOD, AND ANTI-VIRAL MATERIAL UTILIZING HYDROXYL RADICAL GENERATED BY THE METHOD | MOCHIGASE CO., LTD. | 8-
Feb- 08 |
9-
Feb -09 |
30-
Dec -10 |
| US20100319
074 |
MULTI-TARGETED RNAI THERAPEUTICS FOR SCARLESS WOUND HEALING OF SKIN | SIR NAOMICS, INC. | 6-
Nov- 07 |
6-
Nov -08 |
16-
Dec -10 |
| US20100317
684 |
Amide and Carbamate Derivatives of N-{2-[4-Amino-2- (Ethoxymethyl)-1H-Imidazo[4,5-c] Quinolin-1-Yl]-1,1-
Dimethylethyl} Methanesulfonamide and Methods |
Coley Pharmaceutical Group, Inc. | 9-
Sep- 05 |
8-
Sep -06 |
16-
Dec -10 |
| US20100317
601 |
Casein Derived Peptides And Uses Thereof | SIDELMAN ZVI | 1-
Mar- 00 |
28-
Jul- 10 |
16-
Dec -10 |
| US20100317
054 |
PORCINE DC-SIGN, ICAM-3 AND LSECtin AND USES THEREOF | VIRGINIA TECH INTELLECTUAL PROPERTIES, INC. | 29-
Oct- 07 |
29-
Oct -08 |
16-
Dec -10 |
| US20100317
034 |
PRIMATE T-LYMPHOTROPIC VIRUSES | and Johns Hopkins University | 21-
Feb- 05 |
1-
Jul- 10 |
16-
Dec -10 |
| US20100316
695 |
CONDENSATION PRODUCTS BASED ON BICYCLIC OR POLYCYCLIC AROMATICS OR HETEROAROMATICS | BASF SE | 14-
Nov- 07 |
11-
Nov -08 |
16-
Dec -10 |
| US20100316
641 |
ENGINEERED ANTIBODY CONSTANT DOMAIN MOLECULES | DIMITROV DIMITER S | 31-
Jan- 08 |
30-
Jan -09 |
16-
Dec -10 |
| US20100316
624 |
TREATMENT OF FIBROSES AND LIVER DISORDERS | LOIBNER HANS | 21-
Dec- 07 |
22-
Dec -08 |
16-
Dec -10 |
| US20100311
797 |
Novel Compounds | BONNERT ROGER VICTOR | 26-
May- 06 |
2-
Jun -10 |
9-
Dec -10 |
| US20100311
656 |
TREATMENT OR PREVENTION OF RESPIRATORY VIRAL INFECTIONS WITH ALPHA THYMOSIN PEPTIDES | SciClone Pharmaceuticals, Inc. | 23-
Apr- 03 |
16-
Jun -10 |
9-
Dec -10 |
| US20100311
039 |
NON-TARGET AMPLIFICATION METHOD FOR DETECTION OF RNA SPLICE-FORMS IN A SAMPLE | QIAGEN GAITHERSBURG INC. | 1-
May- 09 |
30-
Apr -10 |
9-
Dec -10 |
| US20100310
638 |
LIPOSOMES COMPRISING DURAMYCIN AND ANTI-VIRAL AGENTS | Board of Regents, The University of Texas System | 15-
Jul-02 |
4-
Mar -10 |
9-
Dec -10 |
| US20100310
604 |
RECOMBINANT INFLUENZA VIRUS-LIKE PARTICLES (VLPS) PRODUCED IN TRANSGENIC PLANTS EXPRESSING HEMAGGLUTININ | MEDICAGO INC. | 21-
Jan- 08 |
12-
Jan -09 |
9-
Dec -10 |
| US20100310
562 |
SYSTEM FOR DELIVERY INTO XCR1 POSITIVE CELL AND USES THEREOF | Bundesrepublik Deutschland letztvertreten durch das Robert Koch-Institut vertreten durch seinen | 20-
Nov- 07 |
19-
Nov -08 |
9-
Dec -10 |
| US20100305
138 |
PYRIDYL DERIVATIVES AND THEIR USE AS THERAPEUTIC AGENTS | XENON PHARMACEUTICALS INC. | 29-
Jul-03 |
15-
Jun -10 |
2-
Dec -10 |
| US20100304
363 |
PEPTIDE COMPOUNDS FOR DETECTING OR INHIBITING SARS CORONAVIRUS AND APPLICATION THEREOF | ELECTRONICS AND TELECOMMUNICATIONS RESEARCH INSTITUTE | 27-
May- 09 |
26-
May -10 |
2-
Dec -10 |
| US20100304
361 |
METHOD AND DEVICE FOR MONITORING A THERAPEUTIC TREATMENT REGIME | DAS DEVELOPMENT PARTNERS | 26-
Jul-07 |
28-
Jul- 08 |
2-
Dec -10 |
| US20100303
823 |
HUMAN MONOCLONAL ANTIBODIES AGAINST INTERLEUKIN 8 (IL-8) | GENMAB A/S | 16-
Dec- 02 |
11-
Nov -09 |
2-
Dec -10 |
| US20100298
547 |
METHODS FOR THE DIRECTED EXPANSION OF EPITOPES FOR USE AS ANTIBODY LIGANDS | BONNIN DUSTAN | 7-
May- 07 |
7-
May -08 |
25-
Nov -10 |
| US20100298
364 |
SALTS 756 | AstraZeneca AB | 21-
May- 09 |
20-
May -10 |
25-
Nov -10 |
| US20100298
209 |
COMPOUNDS FOR PREVENTING OR TREATING A VIRAL INFECTION | ESTAQUIER JEROME | 2-
Nov- 07 |
31-
Oct -08 |
25-
Nov -10 |
| US20100297
651 |
Methods of Modulating Vesicular Trafficking | THE GENERAL HOSPITAL CORPORATION | 21-
May- 09 |
21-
May -10 |
25-
Nov -10 |
| US20100297
165 |
IMMUNOSTIMULATORY COMBINATIONS OF TLR LIGANDS AND METHODS OF USE | Office of Technology Transfer | 24-
Sep- 07 |
24-
Sep -08 |
25-
Nov -10 |
| US20100297
108 |
METHOD OF PROVIDING PATIENT SPECIFIC IMMUNE RESPONSE IN AMYLOIDOSES AND PROTEIN AGGREGATION DISORDERS | GRIMM JAN | 31-
Aug- 07 |
1-
Sep -08 |
25-
Nov -10 |
| US20100292
504 |
BORON-CONTAINING SMALL MOLECULES | Anacor Pharmaceuticals, Inc. | 20-
Jun- 07 |
30-
Jul- 10 |
18-
Nov -10 |
| US20100292
307 |
Construct | ISIS INNOVATION LIMITED | 28-
Mar- 06 |
28-
Mar -07 |
18-
Nov -10 |
| US20100291
544 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF STRAINS OF HEPATITIS C VIRUS | IBIS BIOSCIENCES, INC. | 25-
May- 07 |
27-
May -08 |
18-
Nov -10 |
| US20100291
145 |
Ii-KEY/ANTIGENIC EPITOPE HYBRID PEPTIDE VACCINES | ANTIGEN EXPRESS, INC. | 14-
Sep- 99 |
26-
Jul- 10 |
18-
Nov -10 |
| US20100291
129 |
Norovirus and sapovirus antigens | COIT DORIS | 22-
Nov- 05 |
24-
Mar -09 |
18-
Nov -10 |
| US20100291
120 |
PEPTIDES REGULATING THE SURFACE EXPRESSION OF THE T CELL RECEPTOR | MAX-DELBRUCK-CENTRUM FUR MOLEKULARE MEDIZIN | 23-
Jun- 06 |
23-
Jun -07 |
18-
Nov -10 |
| US20100291
075 |
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Neutralizing Antibodies | Theraclone Sciences, Inc. | 23-
Apr- 09 |
23-
Apr -10 |
18-
Nov -10 |
| US20100291
033 |
Albumin Fusion Proteins | Human Genome Sciences, Inc. | 21-
Dec- 01 |
3-
Jun -10 |
18-
Nov -10 |
| US20100286
251 |
Mammalian Genes Involved In Viral Infection And Tumor Suppression | RUBIN DONALD H | 11-
Oct- 06 |
5-
Mar -10 |
11-
Nov -10 |
| US20100286
238 |
SUPPRESSION OF VIRUSES INVOLVED IN RESPIRATORY INFECTION OR DISEASE | ARNDT GREGORY MARTIN | 15-
May- 07 |
14-
May -08 |
11-
Nov -10 |
| US20100286
118 |
SUBSTITUTED 1-CYANOETHYLHETEROCYCLYLCARBOXAMIDE COMPOUNDS 750 | FORD RHONAN | 7-
May- 09 |
6-
May -10 |
11-
Nov -10 |
| US20100286
025 |
SYNTHETIC APOLIPOPROTEIN E MIMICKING POLYPEPTIDES AND METHODS OF USE | ANANTHARAMAIAH GATTADAHALLI M | 28-
Aug- 07 |
27-
Aug -08 |
11-
Nov -10 |
| US20100285
457 |
High-Throughput Diagnostic Assay For the Human Virus Causing Severe Acute Respiratory Syndrome (SARS) | CHAN KWOK HUNG | 24-
Mar- 03 |
12-
Jun -09 |
11-
Nov -10 |
| US20100285
135 |
Nanoparticles For Use In Immunogenic Compositions | NOVARTIS AG | 2-
Dec- 05 |
1-
Dec -06 |
11-
Nov -10 |
| US20100284
968 |
Use of PS20/WFDC1 and Interferons to Diagnose, Monitor and Treat Viral Diseases | BAIG EHTESHAM | 15-
Oct- 07 |
15-
Oct -08 |
11-
Nov -10 |
| US20100284
967 |
MODIFIED PHAGE FOR DISPLAYING POST-TRANSLATIONALLY MODIFIED PROTEINS AND USES THEREOF | National Institutes of Health (NIH), U.S. Dept. of Health and Human Services (DHHS) U.S. Govt. | 21-
Nov- 06 |
20-
Nov -07 |
11-
Nov -10 |
| US20100284
965 |
COMPOSITIONS AND METHODS FOR ADOPTIVE AND ACTIVE IMMUNOTHERAPY | Yale University | 15-
Jan- 08 |
14-
Jan -09 |
11-
Nov -10 |
| US20100284
917 |
COMPOUNDS AND MARKERS FOR SURFACE-ENHANCED RAMAN SCATTERING | JULIUS-MAXIMILIANS- UNIVERSITAT | 24-
Sep- 07 |
24-
Sep -08 |
11-
Nov -10 |
| US20100284
016 |
OPTICAL CYTOMETRY | THE REGENTS OF THE UNIVERSITY OF CALIFORNIA | 6-
May- 09 |
6-
May -09 |
11-
Nov -10 |
| US20100280
102 |
DOUBLE-STRANDED RIBONUCLEIC ACID WITH INCREASED EFFECTIVENESS IN AN ORGANISM | Alnylam Pharmaceuticals | 13-
Jun- 03 |
16-
Jun -10 |
4-
Nov -10 |
| US20100280
067 |
INHIBITORS OF ACETYL-COA CARBOXYLASE | ACHARYA VINOD PARAMESHWARAN | 30-
Apr- 09 |
30-
Apr -10 |
4-
Nov -10 |
| US20100280
048 |
Some 2-pyrazinone derivatives and their use as inhibitors of neutrophile elastase | ASTRAZENECA R&D | 6-
Nov- 07 |
5-
Nov -08 |
4-
Nov -10 |
| US20100280
001 |
IMIDAZOQUINOLINES WITH IMMUNO-MODULATING PROPERTIES | BONNERT ROGER VICTOR | 8-
May- 07 |
6-
May -08 |
4-
Nov -10 |
| US20100279
906 |
FORMULATIONS COMPRISING AN ANTI-MICROBIAL COMPOSITION | BYOTROL PLC | 17-
Sep- 07 |
17-
Sep -08 |
4-
Nov -10 |
| US20100279
888 |
COMPOSITIONS AND METHODS OF DETECTION | GENERA BIOSYSTEMS LIMITED | 5-
Apr- 07 |
4-
Apr -08 |
4-
Nov -10 |
| US20100279
351 |
Method for Generation of Antibodies | NATIONAL JEWISH MEDICAL AND RESEARCH CENTER | 13-
Mar- 07 |
13-
Mar -08 |
4-
Nov -10 |
| US20100279
279 |
Compositions and methods for analysis of target analytes | DANIELZADEH ROBERT | 17-
Sep- 03 |
30-
Nov -07 |
4-
Nov -10 |
| US20100279
276 |
METHODS FOR DETERMINING THE PRESENCE OF SARS CORONAVIRUS IN A SAMPLE | GEN-PROBE INCORPORATED | 17-
Apr- 03 |
6-
Jul- 10 |
4-
Nov -10 |
| US20100279
273 |
NUCLEIC ACID SEQUENCES FOR THE AMPLIFICATION AND DETECTION OF RESPIRATORY VIRUSES | UNIVERSITE LAVAL | 17-
Jul-07 |
17-
Jul- 08 |
4-
Nov -10 |
| US20100278
861 |
METHODS AND COMPOSITIONS FOR PREDICTING EMERGENCE AND EXPANSION OF DRUG RESISTANT STRAINS OF INFLUENZA VIRUS | NIMAN HENRY L | 29-
Jan- 09 |
29-
Jan -10 |
4-
Nov -10 |
| US20100278
846 |
NASAL-ADMINISTERED VACCINES USING MULTI-SCREENED NALT- TARGETING AND PHAGOCYTIC POLYPEPTIDE TRANSPORT SEQUENCES | FERGUSON IAN A | 23-
Jun- 04 |
11-
Dec -09 |
4-
Nov -10 |
| US20100278
829 |
DUAL INHIBITION OF IMMUNOPHILIN/CYCLOPHILIN FAMILY MEMBERS AND EMMPRIN IMMUNOGLOBULIN RECEPTOR SUPERFAMILY MEMBERS | EDWARDS III CARL KEITH | 6-
Feb- 09 |
6-
Feb -10 |
4-
Nov -10 |
| US20100273
997 |
Ribozyme to cleave coronavirus gene | FUKUDA NOBORU | 9-
Aug- 05 |
9-
Aug -06 |
28-
Oct- 10 |
| US20100273
854 |
COMPOSITIONS AND METHODS FOR INHIBITING NADPH OXIDASE EXPRESSION | FEINSTEIN ELENA | 15-
Jun- 07 |
12-
Jun -08 |
28-
Oct- 10 |
| US20100273
740 |
Phospholipids for the Treatment of Infection by Togaviruses, Herpes Viruses and Coronaviruses | FLEMING RONALD A | 20-
Feb- 04 |
23-
Nov -09 |
28-
Oct- 10 |
| US20100273
670 |
DETECTION OF ANTIVIRAL RESISTANCE IN INFLUENZA A USING DNA MICROARRAY | DAWSON ERICA | 21-
Aug- 06 |
21-
Aug -07 |
28-
Oct- 10 |
| US20100272
785 |
RNA SEQUENCE MOTIFS IN THE CONTEXT OF DEFINED INTERNUCLEOTIDE LINKAGES INDUCING SPECIFIC IMMUNE MODULATORY PROFILES | COLEY PHARMACEUTICAL GMBH | 13-
Aug- 07 |
8-
Aug -08 |
28-
Oct- 10 |
| US20100272
776 |
Device including bone cage and method for treatment of disease in a subject | Searete LLC, a limited liability corporation of the State of Delaware | 23-
Apr- 09 |
28-
Jul- 09 |
28-
Oct- 10 |
| US20100272
771 |
Device including bone cage and method for treatment of disease in a subject | HARLOW ED | 23-
Apr- 09 |
23-
Apr -09 |
28-
Oct- 10 |
| US20100272
730 |
Antigenic GM-CSF Peptides And Antibodies To GM-CSF | MORPHOTEK, INC. | 8-
Feb- 06 |
16-
Apr -10 |
28-
Oct- 10 |
| US20100272
706 |
ANTIVIRALS | GREBER URS | 22-
Jun- 07 |
20-
Jun -08 |
28-
Oct- 10 |
| US20100272
668 |
ANTIVIRAL SUBSTANCE, ANTIVIRAL FIBER, AND ANTIVIRAL FIBER
STRUCTURE |
Daiwabo Holdings Co., Ltd. | 20-
Feb- 08 |
20-
Feb -09 |
28-
Oct- 10 |
| US20100267
981 |
BORON-CONTAINING SMALL MOLECULES | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
2-
Dec -09 |
21-
Oct- 10 |
| US20100267
805 |
TARGETING OPPOSITE STRAND REPLICATION INTERMEDIATES OF SINGLE-STRANDED VIRUSES BY RNAI | ALNYLAM PHARMACEUTICALS, INC. | 24-
Sep- 04 |
1-
Mar -10 |
21-
Oct- 10 |
| US20100266
677 |
NUCLEIC ACID BINDING COMPOUNDS AND METHODS OF USE | UNIVERSITY OF ROCHESTER | 26-
Jul-07 |
28-
Jul- 08 |
21-
Oct- 10 |
| US20100266
634 |
ADJUVANCY AND IMMUNE POTENTIATING PROPERTIES OF NATURAL PRODUCTS OF ONCHOCERCA VOLVULUS | NEW YORK BLOOD CENTER, INC. | 15-
Jun- 04 |
18-
Feb -10 |
21-
Oct- 10 |
| US20100266
620 |
Immunoconjugates for treatment of infectious diseases | SUN LE | 20-
Jan- 06 |
19-
Jan -07 |
21-
Oct- 10 |
| US20100266
604 |
USE OF TAM RECEPTOR INHIBITORS AS IMMUNOENHANCERS AND TAM ACTIVATORS AS IMMUNOSUPPRESSORS | The Salk Institute for Biological Studies | 9-
Nov- 07 |
7-
Nov -08 |
21-
Oct- 10 |
| US20100266
598 |
PEPTIDE THAT ELICITS NEUTRALIZING ANTIBODIES TARGETING THE HIV CO-RECEPTOR, CCR5 | Government of the US, as Represented by the Secretary, Department of Health and Human Services | 9-
Apr- 04 |
28-
Dec -09 |
21-
Oct- 10 |
| US20100262
374 |
Systems and methods for analyzing nanoreporters | HWANG JENQ-NENG | 22-
May- 06 |
21-
May -07 |
14-
Oct- 10 |
| US20100261
779 |
Phosphate-Modified Oligonucleotide Analogs with Enhanced Immunostimulatory Activity | JURK MARION | 15-
May- 08 |
15-
May -08 |
14-
Oct- 10 |
| US20100261
690 |
NOVEL COMBINATIONS | ASTRAZENECA R&D | 3-
Apr- 09 |
30-
Mar -10 |
14-
Oct- 10 |
| US20100261
026 |
Compositions comprising oriented, immobilized macromolecules and methods for their preparation | NanoString Technologies, Inc. | 23-
Dec- 05 |
22-
Dec -06 |
14-
Oct- 10 |
| US20100256
348 |
Oligoadenylate Synthetase (OAS) | ILLUMIGEN BIOSCIENCES, INC. | 23-
Nov- 05 |
30-
Mar -10 |
7-
Oct- 10 |
| US20100256
105 |
NOVEL COMPOUNDS | AstraZeneca R&D | 3-
Apr- 09 |
30-
Mar -10 |
7-
Oct- 10 |
| US20100256
104 |
NOVEL AMIDE COMPOUNDS | ASTRAZENECA R&D | 3-
Apr- 09 |
30-
Mar -10 |
7-
Oct- 10 |
| US20100256
103 |
NOVEL COMPOUNDS | ASTRAZENECA R&D | 3-
Apr- 09 |
30-
Mar -10 |
7-
Oct- 10 |
| US20100256
096 |
Sulfonyl semicarbazides, carbonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses | BAILEY THOMAS R | 6-
Dec- 04 |
17-
Jan -07 |
7-
Oct- 10 |
| US20100256
070 |
RECOMBINANT VIBRIO CHOLERAE EXOTOXINS | SEED BRIAN | 20-
Jul-07 |
18-
Jul- 08 |
7-
Oct- 10 |
| US20100254
985 |
Protein Formulations | MEDIMMUNE, LLC | 3-
Feb- 06 |
6-
May -10 |
7-
Oct- 10 |
| US20100254
944 |
Albumin Fusion Proteins | STUMP DAVID CARTER | 14-
Sep- 06 |
13-
Sep -07 |
7-
Oct- 10 |
| US20100254
942 |
HEPATITIS C ANTIVIRAL COMPOSITIONS AND METHODS | BIOTRON LIMITED | 3-
Aug- 07 |
4-
Aug -08 |
7-
Oct- 10 |
| US20100249
200 |
Novel Compounds 569 | CONNOLLY STEPHEN | 20-
Dec- 06 |
30-
Mar -10 |
30-
Sep -10 |
| US20100249
021 |
METHOD AND MEDICAMENT FOR INHIBITING THE INFECTION OF INFLUENZA VIRUS | INSTITUTE OF MICROBIOLOGY, CHINESE ACADEMY OF SCIENCES | 14-
Nov- 07 |
18-
Dec -07 |
30-
Sep -10 |
| US20100248
219 |
Compositions for use in identification of influenza viruses | ESHOO MARK W | 17-
Oct- 05 |
17-
Oct -06 |
30-
Sep -10 |
| US20100247
574 |
CHIMERIC NEWCASTLE DISEASE VIRUS VLPs | MAHMOOD KUTUB | 21-
Feb- 07 |
21-
Feb -08 |
30-
Sep -10 |
| US20100247
571 |
METHODS AND COMPOSITIONS FOR IMMUNIZATION AGAINST VIRUS | ACADEMIA SINICA | 27-
Mar- 09 |
26-
Mar -10 |
30-
Sep -10 |
| US20100247
560 |
MUTANT BOTULINUM NEUROTOXIN SEROTYPE A POLYPEPTIDE AND USES THEREOF | THOMAS JEFFERSON UNIVERSITY | 25-
Sep- 07 |
25-
Sep -08 |
30-
Sep -10 |
| US20100247
554 |
USE OF TAM RECEPTOR INHIBITORS AS ANTIMICROBIALS | BHATTACHARYYA SUCHITA | 9-
Nov- 07 |
7-
Nov -08 |
30-
Sep -10 |
| US20100247
550 |
SYSTEMS AND METHODS FOR IDENTIFYING REPLIKIN SCAFFOLDS AND USES OF SAID REPLIKIN SCAFFOLDS | BOGOCH ELENORE S | 28-
Apr- 04 |
3-
Feb -10 |
30-
Sep -10 |
| US20100240
903 |
CRYSTALLINE TRIPEPTIDE EPOXY KETONE PROTEASE INHIBITORS | Onyx Therapeutics, Inc. | 20-
Mar- 09 |
22-
Mar -10 |
23-
Sep -10 |
| US20100240
746 |
STEREOISOMERS OF TRICYCLODECAN-9-YL-XANTHOGENATE | LUMAVITA AG | 3-Jul-
07 |
3-
Jul- 08 |
23-
Sep -10 |
| US20100240
693 |
Oxime and Hydroxylamine Substituted Thiazolo [4,5-C] Ring Compounds and Methods | Coley Pharmaceutical Group, Inc | 9-
Feb- 05 |
8-
Feb -06 |
23-
Sep -10 |
| US20100240
623 |
8-OXOADENINE DERIVATIVES ACTING AS MODULATORS OF TLR7 | COOK ANTHONY | 5-Jul-
06 |
3-
Jul- 07 |
23-
Sep -10 |
| US20100240
063 |
SYSTEMS AND METHODS FOR DETECTING MULTIPLE OPTICAL SIGNALS | GEN-PROBE INCORPORATED | 10-
Mar- 05 |
1-
Jun -10 |
23-
Sep -10 |
| US20100239
611 |
Combination adjuvant formulation | BABIUK LORNE | 16-
Oct- 08 |
15-
Oct -09 |
23-
Sep -10 |
| US20100239
610 |
INFLUENZA VIRUS-LIKE PARTICLES (VLPS) COMPRISING HEMAGGLUTININ PRODUCED WITHIN A PLANT | MEDICAGO INC. | 13-
Jul-07 |
11-
Jul- 08 |
23-
Sep -10 |
| US20100239
593 |
RSV-SPECIFIC BINDING MOLECULES AND MEANS FOR PRODUCING THEM | MedImmune Limited | 1-
Jun- 07 |
30-
May -08 |
23-
Sep -10 |
| US20100239
520 |
ORGANIC COMPOUNDS | DALES NATALIE | 24-
Aug- 06 |
22-
Aug -07 |
23-
Sep -10 |
| US20100234
355 |
ISOQUINOLINE DERIVATIVES AND THEIR USE AS INHIBITORS OF CYTOKINE MEDIATED DISEASES | MARTIN BARRIE | 19-
Jun- 06 |
18-
Jun -07 |
16-
Sep -10 |
| US20100233
250 |
VACCINE | BARAS BENOIT | 19-
Jun- 07 |
20-
Jun -08 |
16-
Sep -10 |
| US20100233
146 |
Coatings and Surface Treatments Having Active Enzymes and Peptides | REACTIVE SURFACES, LTD. | 9-
Sep- 02 |
29-
May -09 |
16-
Sep -10 |
| US20100233
116 |
ORGANIC COMPOUNDS | DALES NATALIE | 15-
Aug- 06 |
13-
Aug -07 |
16-
Sep -10 |
| US20100233
084 |
Method for Delivery Across the Blood Brain Barrier | IMMUNE DISEASE INSTITUTE, INC. | 22-
May- 06 |
22-
May -07 |
16-
Sep -10 |
| US20100233
031 |
Method and Apparatus for Analyzing Bioprocess Fluids | BioScale, Inc. | 2-
May- 05 |
25-
May -10 |
16-
Sep -10 |
| US20100227
767 |
STOCHASTIC CONFINEMENT TO DETECT, MANIPULATE, AND UTILIZE MOLECULES AND ORGANISMS | BOEDICKER JAMES Q | 26-
Jul-07 |
28-
Jul- 08 |
9-
Sep -10 |
| US20100226
931 |
Compounds for immunopotentiation | SILVER JOEL B | 24-
Jun- 04 |
24-
Jun -05 |
9-
Sep -10 |
| US20100222
409 |
COMPOSITIONS AND METHODS FOR INHIBITING EXPRESSION OF PRO-APOPTOTIC GENES | FEINSTEIN ELENA | 27-
Jun- 07 |
26-
Jun -08 |
2-
Sep -10 |
| US20100222
234 |
Malaria antigen screening method | AGUAIR JOAO CARLOS | 31-
Aug- 05 |
25-
Aug -06 |
2-
Sep -10 |
| US20100221
755 |
USE OF ANTIBODY SECRETING CELL ELISPOT TO ASSESS ANTIBODY RESPONSES FOLLOWING ANTIGEN EXPOSURE | University of Rochester | 15-
Jun- 07 |
16-
Jun -08 |
2-
Sep -10 |
| US20100221
349 |
NUCLEIC ACID CONSTRUCTS | Powderject Vaccines, Inc. | 1-
Feb- 05 |
1-
Feb -06 |
2-
Sep -10 |
| US20100221
307 |
ANTIVIRAL AGENTS, ANTIVIRAL FIBERS AND ANTIVIRAL FIBER STRUCTURES | DAIWABO HOLDINGS CO., LTD. | 20-
Feb- 08 |
20-
Feb -09 |
2-
Sep -10 |
| US20100221
257 |
BINDING MEMBERS-513 | MEDIMMUNE LIMITED | 7-
Nov- 08 |
5-
Nov -09 |
2-
Sep -10 |
| US20100221
242 |
ANTI-VIRAL GRIFFITHSIN COMPOUNDS, COMPOSITIONS AND METHODS OF USE | MCMAHON JAMES B | 1-
Dec- 05 |
1-
Dec -06 |
2-
Sep -10 |
| US20100216
843 |
NOVEL SALT 628 | ASTRAZENECA R&D | 20-
Feb- 09 |
16-
Feb -10 |
26-
Aug -10 |
| US20100216
773 |
PHENANTHROINDOLIZIDINE ANALOGUES | National Health Research Institutes | 24-
Feb- 09 |
11-
Feb -10 |
26-
Aug -10 |
| US20100216
689 |
CYTOTOXIC T CELL ACTIVATOR COMPRISING EP4 AGONIST | National University Corporation, Hamamatsu University School of Medicine | 8-
May- 07 |
7-
May -08 |
26-
Aug -10 |
| US20100215
683 |
PROTECTIVE ANTIGENS FOR GROUP B STREPTOCOCCUS HYPERVIRULENT STRAINS | NOVARTIS AG | 20-
Feb- 09 |
16-
Feb -10 |
26-
Aug -10 |
| US20100215
675 |
REPLIKIN-BASED COMPOUNDS FOR PREVENTION AND TREATMENT OF INFLUENZA AND METHODS OF DIFFERENTIATING INFECTIVITY AND LETHALITY IN INFLUENZA | BOGOCH ELENORE S | 9-
Jan- 09 |
16-
Oct -09 |
26-
Aug -10 |
| US20100210
745 |
Molecular Healing of Polymeric Materials, Coatings, Plastics, Elastomers, Composites, Laminates, Adhesives, and Sealants by Active Enzymes | REACTIVE SURFACES, LTD. | 9-
Sep- 02 |
29-
Jan -10 |
19-
Aug -10 |
| US20100210
688 |
Novel Benzothiazolone Derivatives | BONNERT ROGER | 9-
Aug- 05 |
27-
Apr -10 |
19-
Aug -10 |
| US20100209
454 |
ATTENUATED VIRUSES USEFUL FOR VACCINES | CELLO JERONIMO | 30-
Mar- 07 |
31-
Mar -08 |
19-
Aug -10 |
| US20100209
451 |
Compositions and Methods Related to Adenovirus Based Delivery of Antigens | INTROGEN THERAPEUTICS, INC. | 16-
Jun- 06 |
18-
Jun -07 |
19-
Aug -10 |
| US20100209
440 |
Targeted Delivery of siRNA | Immune Disease Institute, Inc. | 26-
Jan- 07 |
25-
Jan -08 |
19-
Aug -10 |
| US20100204
286 |
METHOD FOR REDUCING GASTROINTESTINAL ADVERSE EFFECTS OF CYTOTOXIC AGENTS | BYRNES JOHN J | 12-
Feb- 09 |
11-
Feb -10 |
12-
Aug -10 |
| US20100204
266 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF MIXED POPULATIONS OF BIOAGENTS | Ibis Biosciences, INC | 23-
Mar- 07 |
21-
Mar -08 |
12-
Aug -10 |
| US20100204
203 |
Chemical Compounds 637: Pyridopyrimidinediones as PDE4 Inhibitors | BONNERT ROGER VICTOR | 11-
Jan- 07 |
10-
Jan -08 |
12-
Aug -10 |
| US20100203
582 |
Protein Expression System Involving Mutated Severe Respiratory Syndrome-Associated Coronavirus 3C-Like Protease | Academia Sinica | 12-
Feb- 09 |
12-
Feb -09 |
12-
Aug -10 |
| US20100197
748 |
ANTI-MICROBIAL COMPOSITION | BYOTROL PLC | 17-
Jul-07 |
17-
Jul- 08 |
5-
Aug -10 |
| US20100197
708 |
INDOLE COMPOUNDS | BARDEN TIMOTHY | 7-
Aug- 06 |
7-
Aug -07 |
5-
Aug -10 |
| US20100196
285 |
Use of Deuterium Oxide to Treat Virus-Based Diseases of the Respiratory Tract | BAYERL THOMAS | 7-
Jan- 09 |
6-
Jan -10 |
5-
Aug -10 |
| US20100196
222 |
AIR CLEANING APPARATUS | FUJIFILM CORPORATION | 20-
Sep- 07 |
19-
Sep -08 |
5-
Aug -10 |
| US20100190
748 |
BORON-CONTAINING SMALL MOLECULES | Anacor Pharmaceuticals, Inc. | 16-
Feb- 05 |
21-
Jul- 09 |
29-
Jul- 10 |
| US20100189
772 |
Compositions of TLR ligands and antivirals | COLEY PHARMACEUTICAL GMBH | 27-
Sep- 06 |
27-
Sep -07 |
29-
Jul- 10 |
| US20100186
680 |
LIVESTOCK STERILIZING METHOD, LIVESTOCK STERILIZING APPARATUS, AND LIVESTOCK OR LIVESTOCK MEAT | HAGIWARA NOBUKO | 21-
Feb- 05 |
21-
Feb -06 |
29-
Jul- 10 |
| US20100184
663 |
Methods and Compositions Related to Inhibition of Viral Entry | University of Utah Research Foundation | 8-
Feb- 07 |
8-
Feb -08 |
22-
Jul- 10 |
| US20100184
613 |
Proteome Epitope Tags and Methods of Use Thereof in Protein Modification Analysis | Millipore Corporation | 10-
May- 02 |
9-
Oct -09 |
22-
Jul- 10 |
| US20100184
192 |
AVIAN INFLUENZA CHIMERIC VLPS | PUSHKO PETER | 11-
Jul-03 |
19-
Jan -10 |
22-
Jul- 10 |
| US20100184
190 |
Influencing viral lipid constituents | MedImmune, LLC | 17-
Oct- 06 |
9-
Oct -07 |
22-
Jul- 10 |
| US20100184
019 |
Novel Human Virus Causing Severe Acute Respiratory Syndrome (SARS) and Uses Thereof | CHAN KWOK HUNG | 24-
Mar- 03 |
10-
Feb -10 |
22-
Jul- 10 |
| US20100183
673 |
METHODS AND COMPOSITIONS FOR PRODUCING AN ADENOVIRUS VECTOR FOR USE WITH MULTIPLE VACCINATIONS | ETUBICS CORPORATION | 2-Jul-
07 |
4-
Jan -10 |
22-
Jul- 10 |
| US20100183
671 |
LOW-ADDITIVE INFLUENZA VACCINES | Novartis Vaccines & Diagnostics GmbH & Co., KG | 27-
Jun- 07 |
27-
Jun -08 |
22-
Jul- 10 |
| US20100183
634 |
MULTIFUNCTIONAL NUCLEIC ACID NANO-STRUCTURES | Cornell University, a New York Corporation | 1-
Jan- 09 |
31-
Dec -09 |
22-
Jul- 10 |
| US20100183
516 |
SELF COUPLING RECOMBINANT ANTIBODY FUSION PROTEINS | BARTH STEFAN | 25-
Jul-07 |
25-
Jul- 08 |
22-
Jul- 10 |
| US20100179
145 |
PHARMACEUTICAL COMBINATIONS | GALLAGHER NEIL JAMES | 12-
Oct- 06 |
12-
Oct -07 |
15-
Jul- 10 |
| US20100178
299 |
METHODS AND COMPOSITIONS FOR IMPROVING IMMUNE RESPONSES | Northeastern University | 13-
Feb- 07 |
13-
Feb -08 |
15-
Jul- 10 |
| US20100175
006 |
SYSTEM AND METHOD FOR DETECTING, COLLECTING, ANALYZING, AND COMMUNICATING EVENT-RELATED INFORMATION | Georgetown University | 28-
Aug- 08 |
2-
Dec -09 |
8-
Jul- 10 |
| US20100173
980 |
ANTIVIRAL OLIGONUCLEOTIDES TARGETING RSV | JUTEAU JEAN-MARC | 13-
Sep- 02 |
21-
Dec -09 |
8-
Jul- 10 |
| US20100173
906 |
Substituted 3,4,6,7-Tetrahydro-5H-1,2a,4a,8- Tetraazacyclopenta[cd]Phenalenes and Methods | GRIESGRABER GEORGE W | 6-
Sep- 06 |
6-
Sep -07 |
8-
Jul- 10 |
| US20100173
325 |
Composition and Method for Analysis of Target Analytes | Millipore Corporation | 17-
Sep- 03 |
4-
Jul- 09 |
8-
Jul- 10 |
| US20100173
280 |
Systems for Detection and Production of Respiratory, Herpes and Enteric Viruses | Diagnostic Hybrids, Inc | 20-
Sep- 05 |
19-
Sep -06 |
8-
Jul- 10 |
| US20100172
965 |
ANTIVIRAL OLIGONUCLEOTIDES TARGETING VIRAL FAMILIES | JUTEAU JEAN-MARC | 13-
Sep- 02 |
21-
Dec -09 |
8-
Jul- 10 |
| US20100172
917 |
Binding molecules against SARS-coronavirus and uses thereof | Crucell Holland B.V. | 22-
Jul-03 |
16-
Nov -09 |
8-
Jul- 10 |
| US20100166
874 |
Technology for Preparation of Macromolecular Microspheres | FANG FANG | 24-
Jan- 06 |
8-
Dec -09 |
1-
Jul- 10 |
| US20100166
782 |
CLIP INHIBITORS AND METHODS OF MODULATING IMMUNE FUNCTION | AGADJANYAN MICHAEL | 25-
Jul-08 |
23-
Jul- 09 |
1-
Jul- 10 |
| US20100166
780 |
CpG Oligonucleotide Analogs Containing Hydrophobic T Analogs with Enhanced Immunostimulatory Activity | Pfizer Inc | 27-
Sep- 06 |
27-
Sep -07 |
1-
Jul- 10 |
| US20100166
769 |
High-yield Transgenic Mammalian Expression System for Generating Virus-like Particles | Academia Sinica | 5-
Sep- 06 |
12-
Feb -10 |
1-
Jul- 10 |
| US20100166
661 |
siRNA COMPOSITIONS AND METHODS FOR POTENTLY INHIBITING VIRAL INFECTION | LIN YONGPING | 11-
Dec- 08 |
11-
Dec -09 |
1-
Jul- 10 |
| US20100160
486 |
POLYOLEFIN ANTIMICROBIAL COMPOSITIONS AND MELTPROCESSING METHODS | BISHOP KEVIN L | 19-
Dec- 08 |
19-
Dec -08 |
24-
Jun- 10 |
| US20100160
323 |
NOVEL PIPERAZINE DERIVATIVES AS INHIBITORS OF STEAROYL- CoA DESATURASE | BISCHOFF ALEXANDER | 23-
Dec- 08 |
22-
Dec -09 |
24-
Jun- 10 |
| US20100160
285 |
Biphenyloxyacetic Acid Derivatives for the Treatment of Respiratory Disease | BIRKINSHAW TIMOTHY NICHOLAS | 24-
Aug- 04 |
18-
Dec -09 |
24-
Jun- 10 |
| US20100160
245 |
Quercetin-Containing Compositions | Quercegen Pharma LLC | 17-
Jul-06 |
16-
Dec -09 |
24-
Jun- 10 |
| US20100158
943 |
ADMINISTRATION ROUTES FOR PRIMING/BOOSTING WITH INFLUENZA VACCINES | NOVARTIS VACCINES AND DIAGNOSTICS SRL | 4-
Nov- 05 |
6-
Nov -06 |
24-
Jun- 10 |
| US20100158
935 |
MUTANT FORMS OF STREPTOLYSIN O | NOVARTIS AG | 21-
Dec- 07 |
24-
Feb -10 |
24-
Jun- 10 |
| US20100158
928 |
IMMUNE RESPONSE MODIFIER COMPOSITIONS AND METHODS | GRIESGRABER GEORGE W | 25-
Dec- 06 |
20-
Dec -07 |
24-
Jun- 10 |
| US20100152
230 |
HYDROXY SUBSTITUTED 1H-IMIDAZOPYRIDINES AND METHODS | Pfizer Inc. | 2-
Sep- 05 |
1-
Sep -06 |
17-
Jun- 10 |
| US20100152
197 |
(4-TERT-BUTYLPIPERAZIN-2-YL)(PIPERAZIN-1-YL)METHANONE-N- CARBOXAMIDE DERIVATIVES | ASTRAZENECA AB | 15-
Dec- 08 |
10-
Dec -09 |
17-
Jun- 10 |
| US20100152
187 |
AMINOTHIAZOLE DERIVATIVES AS HUMAN STEAROYL-COA DESATURASE INHIBITORS | XENON PHARMACEUTICALS INC. | 3-
Jun- 05 |
5-
Jun -06 |
17-
Jun- 10 |
| US20100152
184 |
PHARMACEUTICAL COMPOUNDS | ASTEX THERAPEUTICS LIMITED | 16-
Nov- 06 |
12-
Oct -07 |
17-
Jun- 10 |
| US20100151
490 |
Antigenic Protein Conjugates and Process for Preparing Same | ABBOTT LABORATORIES | 1-
Sep- 06 |
25-
Feb -10 |
17-
Jun- 10 |
| US20100150
960 |
COMPOSITIONS AND METHODS FOR CHITOSAN ENHANCED IMMUNE RESPONSE | The United States of America, as represented by the Secretary,Department of Health and Human Servi | 22-
Sep- 06 |
21-
Sep -07 |
17-
Jun- 10 |
| US20100150
943 |
IMMUNOGENIC COMPOSITIONS FOR GRAM POSITIVE BACTERIA | NOVARTIS AG | 26-
Jul-06 |
26-
Jul- 07 |
17-
Jun- 10 |
| US20100150
923 |
FUSION PROTEINS OF RECOMBINANT SARS CORONAVIRUS STRUCTURAL PROTEINS, THEIR PRODUCTION AND USES | Chinese Academy of Medical Sciences, Institute of Basic Medical Sciences | 20-
Jun- 05 |
13-
Jun -06 |
17-
Jun- 10 |
| US20100146
647 |
Antibody producing non-human mammals | Merus B. V. | 27-
Jun- 08 |
19-
Oct -09 |
10-
Jun- 10 |
| US20100144
846 |
Oligoribonucleotides and uses thereof | COLEY PHARMACEUTICAL GMBH | 26-
Oct- 06 |
26-
Oct -07 |
10-
Jun- 10 |
| US20100144
833 |
RNAi Modulation Of RSV, PIV And Other Respiratory Viruses And Uses Thereof | BARIK SAILEN | 22-
Oct- 04 |
25-
Jun -09 |
10-
Jun- 10 |
| US20100144
828 |
CAFFEOYLQUINIC ACID DERIVATIVES CONTAINING NITROGEN, AND PREPARATION METHOD, PHARMACEUTICAL COMPOSITION AND USAGE THEREOF | ZHEJIANG MEDICINE CO., LTD. XINCHANG PHARMACEUTICA | 23-
Mar- 07 |
21-
Mar -08 |
10-
Jun- 10 |
| US20100144
759 |
NOVEL N-(FLUORO-PYRAZINYL)-PHENYLSULFONAMIDES AS MODULATORS OF CHEMOKINE RECEPTOR CCR4 | AstraZeneca AB | 12-
Dec- 05 |
8-
Dec -09 |
10-
Jun- 10 |
| US20100144
606 |
COMBINATION 408 | AstraZeneca AB | 20-
Jun- 08 |
19-
Jun -09 |
10-
Jun- 10 |
| US20100144
589 |
METHODS OF PREDICTING CANCER LETHALITY USING REPLIKIN COUNTS | BOGOCH ELENORE S | 23-
Apr- 08 |
7-
Aug -09 |
10-
Jun- 10 |
| US20100144
040 |
CELLS AND METHODOLOGY TO GENERATE NON-SEGMENTED NEGATIVE-STRAND RNA VIRUSES | CHARNEAU PIERRE | 22-
Dec- 06 |
21-
Dec -07 |
10-
Jun- 10 |
| US20100144
015 |
COMPOSITIONS, METHODS AND USES FOR INDUCING VIRAL GROWTH | KINNEY RICHARD | 5-
Dec- 08 |
4-
Dec -09 |
10-
Jun- 10 |
| US20100143
888 |
ENZYMATIC DIAGNOSTIC TEST FOR SARS AND OTHER VIRAL DISEASES | MND Diagnostic Ltd. | 23-
Jun- 03 |
25-
Nov -09 |
10-
Jun- 10 |
| US20100143
881 |
Selective detection of human rhinovirus | and Human Services | 5-
Dec- 08 |
5-
Dec -08 |
10-
Jun- 10 |
| US20100143
411 |
METHOD FOR IDENTIFICATION OF T-LYMPHOCYTE ANTIGENS | The Government of the U.S.A. as represented by the Secretary, Dept. of Health and Human Service | 27-
May- 08 |
27-
May -09 |
10-
Jun- 10 |
| US20100143
406 |
METHODS OF ENHANCING PROTEIN INCORPORATION INTO VIRUS LIKE PARTICLES | PUSHKO PETER | 30-
Jun- 06 |
27-
Jun -07 |
10-
Jun- 10 |
| US20100143
302 |
Recombinant Adenoviruses Based on Serotype 26 and 48, and Use Thereof | Beth Israel Deaconess Medical Center, Inc | 16-
Mar- 06 |
15-
Mar -07 |
10-
Jun- 10 |
| US20100130
503 |
NEW FLUORENE DERIVATIVES, COMPOSITIONS CONTAINING THE SAME AND USE THEREOF AS INHIBITORS OF THE PROTEIN CHAPERONE HSP 90 | SANOFI-AVENTIS | 24-
Oct- 06 |
24-
Nov -09 |
27-
May -10 |
| US20100130
491 |
9-SUBSTITUTED-8-OXO-ADENINE COMPOUNDS AS TOLL-LIKE RECEPTOR (TLR7 ) MODULATORS | BONNERT ROGER VICTOR | 19-
Mar- 07 |
19-
Mar -08 |
27-
May -10 |
| US20100130
430 |
STABILIZED THERAPEUTIC SMALL HELICAL ANTIVIRAL PEPTIDES | New York Blood Center, Inc | 5-
Oct- 06 |
2-
Oct -07 |
27-
May -10 |
| US20100129
811 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF PSEUDOMONAS AERUGINOSA | Ibis Biosciences, Inc. | 11-
Sep- 03 |
2-
Oct -09 |
27-
May -10 |
| US20100129
437 |
TARGETED INTRACELLULAR DELIVERY OF ANTIVIRAL AGENTS | BBB Holding B.V. | 23-
Mar- 07 |
21-
Mar -08 |
27-
May -10 |
| US20100129
400 |
ATTENUATED VIRUSES, VACCINES AND METHODS OF USE THEREOF | THE PENN STATE RESEARCH FOUNDATION | 22-
Dec- 06 |
12-
Jan -10 |
27-
May -10 |
| US20100129
386 |
Composotions And Methods For The Identification And Treatment Of Immune-Mediated Inflammatory Diseases | CONG YINGZI | 24-
Jan- 06 |
24-
Jan -07 |
27-
May -10 |
| US20100129
321 |
DIARYL UREA FOR TREATING VIRUS INFECTIONS | BAYER HEALTHCARE LLC | 15-
Dec- 05 |
6-
Dec -06 |
27-
May -10 |
| US20100119
550 |
RECOMBINANT MULTIVALENT VACCINE | GOMI YASUYUKI | 24-
Nov- 05 |
24-
Nov -05 |
13-
May -10 |
| US20100113
745 |
Means and Methods for Influencing the Stability of Antibody Producing Cells | Meibergdreef 9 | 9-
Dec- 05 |
8-
Dec -06 |
6-
May -10 |
| US20100113
565 |
IMMUNOSTIMULATORY COMBINATIONS AND METHODS | Not Available | 8-
Dec- 04 |
8-
Dec -05 |
6-
May -10 |
| US20100113
510 |
QUINUCLIDINOL DERIVATIVES AS MUSCARINIC RECEPTOR ANTAGONISTS | FORD RHONAN | 19-
Dec- 06 |
17-
Dec -07 |
6-
May -10 |
| US20100112
710 |
METHODS AND COMPUTER SYSTEMS FOR IDENTIFYING TARGET- SPECIFIC SEQUENCES FOR USE IN NANOREPORTERS | NANOSTRING TECHNOLOGIES, INC. | 10-
Apr- 07 |
10-
Apr -08 |
6-
May -10 |
| US20100112
643 |
METHOD FOR DIRECT CAPTURE OF RIBONUCLEIC ACID | The Government of the United States of America, as represented by the Secretary of the Navy | 4-
Nov- 08 |
4-
Nov -08 |
6-
May -10 |
| US20100112
092 |
ANTIMICROBIAL SOLUTIONS CONTAINING DICHLORINE MONOXIDE AND METHODS OF MAKING AND USING THE SAME | Oculus Innovative Sciences, Inc. | 13-
Mar- 07 |
13-
Mar -08 |
6-
May -10 |
| US20100111
873 |
TREATING CANCER WITH VIRAL NUCLEIC ACID | HADAC ELIZABETH M | 20-
Feb- 07 |
20-
Feb -08 |
6-
May -10 |
| US20100105
873 |
INTEGRATED APPROACH FOR GENERATING MULTIDOMAIN PROTEIN THERAPEUTICS | MEDIMMUNE, INC. | 1-Jul-
05 |
30-
Jun -06 |
29-
Apr- 10 |
| US20100105
642 |
NOVEL COMPOUNDS 409 | ASTRAZENECA AB | 18-
Jun- 08 |
18-
Jun -09 |
29-
Apr- 10 |
| US20100105
025 |
DEVICES FOR GENERATING DETECTABLE POLYMERS | Not Available | 12-
Oct- 06 |
12-
Oct -07 |
29-
Apr- 10 |
| US20100105
024 |
RAPID TEST INCLUDING GENETIC SEQUENCE PROBE | Transgenex Nanobiotech, Inc. | 14-
Jan- 08 |
16-
Apr -09 |
29-
Apr- 10 |
| US20100104
503 |
MODULAR NANOPARTICLES FOR ADAPTABLE VACCINES | CAPLAN MICHAEL J | 15-
Feb- 07 |
15-
Feb -08 |
29-
Apr- 10 |
| US20100099
870 |
NOVEL ADENINE COMPOUND | AstraZeneca Aktiebolag | 20-
Mar- 07 |
19-
Mar -08 |
22-
Apr- 10 |
| US20100099
745 |
ENHANCING DISEASE RESISTANCE AGAINST RNA VIRAL INFECTIONS WITH INTRACYTOPLASMIC PATHOGEN SENSORS | GUO ZHA | 18-
Oct- 06 |
16-
Oct -07 |
22-
Apr- 10 |
| US20100099
672 |
METHODS FOR TREATING HEPATITIS C | PTC THERAPEUTICS, INC. | 22-
Jul-04 |
23-
Dec -09 |
22-
Apr- 10 |
| US20100099
665 |
ISOQUINOLINONE DERIVATIVES | AstraZeneca AB | 1-
Oct- 08 |
1-
Oct -09 |
22-
Apr- 10 |
| US20100099
149 |
STABILIZING COMPOSITIONS AND METHODS FOR EXTRACTION OF RIBONUCLEIC ACID | DNA GENOTEK INC. | 6-
Oct- 06 |
5-
Oct -07 |
22-
Apr- 10 |
| US20100099
079 |
Non-dividing cell-based assay for high throughput antiviral compound screening | SAINZ JR BRUNO | 26-
Sep- 08 |
24-
Sep -09 |
22-
Apr- 10 |
| US20100093
998 |
NOVEL ADENINE COMPOUND | AstraZeneca Aktiebolag | 20-
Mar- 07 |
19-
Mar -08 |
15-
Apr- 10 |
| US20100093
862 |
POTENTIATION OF CELLULAR IMMUNITY USING HISTONE DEACETYLASE (HDAC) INHIBITORS. | Sapporo Medical Univeristy and Japan Science and Technology Agency | 6-
Dec- 06 |
6-
Dec -07 |
15-
Apr- 10 |
| US20100093
851 |
SILVER POLYAMIDE COMPOSITE | BISHOP KEVIN L | 14-
Oct- 08 |
14-
Oct -08 |
15-
Apr- 10 |
| US20100093
816 |
SALT | ARGENTA DISCOVERY LTD. | 7-
Feb- 07 |
6-
Feb -08 |
15-
Apr- 10 |
| US20100093
813 |
Salts 668 | ASTRAZENECA AB | 8-
Feb- 07 |
6-
Feb -08 |
15-
Apr- 10 |
| US20100093
627 |
ALBUMIN FUSION PROTEINS | Human Genome Sciences. Inc. | 9-
Feb- 04 |
3-
Aug -09 |
15-
Apr- 10 |
| US20100093
038 |
Means and Methods for Influencing the Stability of Cells | Academisch Medisch Centrum bij de Universiteit van Amsterdam | 9-
Dec- 05 |
9-
Dec -05 |
15-
Apr- 10 |
| US20100092
474 |
PHARMACEUTICAL COMBINATIONS | GALLAGHER NEIL JAMES | 12-
Oct- 06 |
12-
Oct -07 |
15-
Apr- 10 |
| US20100092
470 |
ANTIBODIES, ANALOGS AND USES THEREOF | ICB International, Inc. | 22-
Sep- 08 |
21-
Sep -09 |
15-
Apr- 10 |
| US20100092
401 |
IMMUNE RESPONSE MODIFIER FORMULATIONS | Graceway Pharmaceuticals, LLC | 18-
Jul-06 |
12-
Jul- 07 |
15-
Apr- 10 |
| US20100092
399 |
METHODS OF TREATING OR PREVENTING INFLAMMATION AND HYPERSENSITIVITY WITH OXIDATIVE REDUCTIVE POTENTIAL
WATER SOLUTION |
Oculus Innovative Sciences, Inc. | 20-
Jan- 06 |
21-
Dec -09 |
15-
Apr- 10 |
| US20100087
443 |
9-SUBSTITUTED-8-OXO-ADENINE COMPOUNDS AS TOLL-LIKE RECEPTOR (TLR7) MODULATORS | Not Available | 19-
Mar- 07 |
19-
Mar -08 |
8-
Apr- 10 |
| US20100087
388 |
ODCASE INHIBITORS AS ANTI-VIRALS AND ANTIBIOTICS | Not Available | 3-
Oct- 05 |
3-
Oct -06 |
8-
Apr- 10 |
| US20100086
950 |
IP-10 BASED IMMUNOLOGICAL MONITORING | Hvidovre Hospital | 5-
Sep- 06 |
5-
Sep -07 |
8-
Apr- 10 |
| US20100086
942 |
COMPOUNDS | AstraZeneca AB | 21-
Feb- 07 |
4-
Dec -09 |
8-
Apr- 10 |
| US20100086
571 |
METHODS TO DECREASE THE RISK OF METABOLIC SYNDROME POST IMMUNIZATION | CLASSEN JOHN BARTHELOW | 5-
Oct- 06 |
5-
Oct -07 |
8-
Apr- 10 |
| US20100075
298 |
Method for rapid identification and quantification of microorganisms | The Regents of the University of California | 23-
Sep- 08 |
23-
Sep -08 |
25-
Mar -10 |
| US20100070
195 |
COMPUTER-IMPLEMENTED BIOLOGICAL SEQUENCE IDENTIFIER SYSTEM AND METHOD | The Government of the United States of America, as represented by the Secretary of the Navy | 2-Jul-
04 |
12-
Nov -09 |
18-
Mar -10 |
| US20100070
194 |
METHODS FOR RAPID IDENTIFICATION AND QUANTITATION OF NUCLEIC ACID VARIANTS | ECKER DAVID J | 21-
Jul-05 |
11-
Nov -09 |
18-
Mar -10 |
| US20100069
614 |
Antibody producing non-human mammals | Merus B.V. | 27-
Jun- 08 |
29-
Jun -09 |
18-
Mar -10 |
| US20100069
427 |
Oxime and Hydroxylamine Substituted Imidazo[4,5-c] Ring Compounds and Methods | COLEY PHARMACEUTICAL GROUP, INC. | 11-
Feb- 05 |
10-
Feb -06 |
18-
Mar -10 |
| US20100068
223 |
Storage of Influenza Vaccines Without Refrigeration | SCHEFFCZIK HANNO | 24-
Mar- 06 |
23-
Mar -07 |
18-
Mar -10 |
| US20100063
126 |
Carbazole-Derived Pharmaceutical Compositions | JadoLabs GmbH | 29-
Jun- 04 |
29-
Jun -05 |
11-
Mar -10 |
| US20100062
441 |
C-MET MUTATIONS AND USES THEREOF | SALGIA RAVI | 15-
Mar- 07 |
14-
Mar -08 |
11-
Mar -10 |
| US20100061
995 |
Immunotherapy To Treat Or Prevent Viral Infection | CARRAGHER DAMIAN MICHAEL | 8-
Sep- 08 |
8-
Sep -08 |
11-
Mar -10 |
| US20100061
961 |
RECOMBINANT SUPER-COMPOUND INTERFERON | WEI GUANGWEN | 28-
Aug- 03 |
4-
Sep -09 |
11-
Mar -10 |
| US20100056
508 |
AMINE DERIVATIVES AND THEIR USE IN BETA-2- ADRENORECEPTOR MEDIATED DISEASES | ASTRAZENECA AB | 20-
Dec- 06 |
19-
Dec -07 |
4-
Mar -10 |
| US20100056
383 |
HIGH DENSITY SELF-CONTAINED BIOLOGICAL ANALYSIS | JONES DAVID E | 15-
Nov- 06 |
14-
Nov -07 |
4-
Mar -10 |
| US20100055
761 |
METHODS, COMPOSITIONS, AND KITS FOR THE SELECTIVE ACTIVATION OF PROTOXINS THROUGH COMBINATORAL TARGETING | THE GENERAL HOSPITAL CORPORATION | 20-
Jul-06 |
20-
Jul- 07 |
4-
Mar -10 |
| US20100055
116 |
Methods and Compositions for Targeting c-Rel | CHENG SHUHUA | 13-
Apr- 06 |
13-
Apr -07 |
4-
Mar -10 |
| US20100048
584 |
PYRIDYL DERIVATIVES AND THEIR USE AS THERAPEUTIC AGENTS | XENON PHARMACEUTICALS INC. | 30-
Jul-03 |
2-
Sep -09 |
25-
Feb -10 |
| US20100048
575 |
NOVEL TETRACYCLIC INHIBITORS OF CYSTEINE PROTEASES, THE PHARMACEUTICAL COMPOSITIONS THEREOF AND THEIR THERAPEUTIC APPLICATIONS | COLLAND FREDERIC | 30-
Oct- 06 |
25-
Oct -07 |
25-
Feb -10 |
| US20100048
472 |
Albumin Fusion Proteins | Human Genome Sciences, Inc. | 22-
Jan- 03 |
20-
Apr -09 |
25-
Feb -10 |
| US20100047
282 |
METHOD OF ACCELERATED VACCINATION AGAINST EBOLA VIRUSES | The Government of the USA as represented by the Secretary of Health and Human Services, NIH | 2-
Aug- 04 |
5-
Nov -09 |
25-
Feb -10 |
| US20100047
277 |
VIROSOMES, METHODS OF PREPARATION, AND IMMUNOGENIC COMPOSITIONS | COMPANS RICHARD W | 13-
Jul-06 |
12-
Jul- 07 |
25-
Feb -10 |
| US20100047
264 |
Immunogenic Affinity-Conjugated Antigen Systems Based on Papaya Mosaic Virus and Uses Thereof | FOLIA BIOTECH INC. | 15-
Nov- 06 |
25-
Oct -07 |
25-
Feb -10 |
| US20100047
179 |
TARGETED SPLIT BIOMOLECULAR CONJUGATES FOR THE TREATMENT OF DISEASES, MALIGNANCIES AND DISORDERS, AND METHODS OF THEIR PRODUCTION | ST. JUDE CHILDREN’S RESEARCH HOSPITAL | 27-
Oct- 06 |
26-
Oct -07 |
25-
Feb -10 |
| US20100042
394 |
System and Method to Predict the Global Spread of Infectious Agents Via Commercial Air Travel | KHAN KAMRAN | 2-
Apr- 07 |
2-
Apr -08 |
18-
Feb -10 |
| US20100041
638 |
Chemical Compounds 293 | ANDREWS GLEN | 27-
May- 08 |
26-
May -09 |
18-
Feb -10 |
| US20100041
617 |
Modulating mxa expression | CHUNG EUN J | 27-
Sep- 04 |
27-
Sep -05 |
18-
Feb -10 |
| US20100041
013 |
ASSAY FOR A HEALTH STATE | HUMAN GENETIC SIGNATURES PTY LTD. | 14-
Sep- 05 |
14-
Sep -06 |
18-
Feb -10 |
| US20100040
703 |
USE OF NITRIC OXIDE | MILLER CHRIS | 13-
Aug- 08 |
12-
Aug -09 |
18-
Feb -10 |
| US20100040
676 |
POLYCISTRONIC HIV VECTOR CONSTRUCTS | Novartis Vaccines and Diagnostics, Inc. | 5-
May- 04 |
22-
Oct -09 |
18-
Feb -10 |
| US20100040
655 |
Anti-viral Formulations Nanomaterials And Nanoparticles | INTRINSIQ MATERIALS LIMITED | 16-
Feb- 06 |
16-
Feb -07 |
18-
Feb -10 |
| US20100040
635 |
NEUTRALIZING ANTIBODIES TO INFLUENZA VIRUSES | Sea Lane Biotechnologies | 28-
Mar- 08 |
27-
Mar -09 |
18-
Feb -10 |
| US20100035
973 |
DISRUPTION OF PROGRAMMED DEATH 1 (PD-1) LIGAND TO ADJUVANT ADENO-ASSOCIATED VIRUS VECTOR VACCINES | Nationwide Children’s Hospital, Inc. | 17-
Jul-06 |
13-
Jul- 07 |
11-
Feb -10 |
| US20100035
848 |
THERAPY FOR DISORDERS OF THE PROXIMAL DIGESTIVE TRACT | BARNES THOMAS MICHAEL | 10-
Mar- 08 |
10-
Mar -09 |
11-
Feb -10 |
| US20100035
836 |
BICYCLIC NUCLEOSIDES AND NUCLEOTIDES AS THERAPEUTIC AGENTS | BETHELL RICHARD | 3-Jul-
08 |
1-
Jul- 09 |
11-
Feb -10 |
| US20100035
761 |
High-throughput rna structure analysis | GIDDINGS MORGAN C | 5-
Jun- 06 |
5-
Jun -07 |
11-
Feb -10 |
| US20100035
239 |
Compositions for use in identification of bacteria | ISIS Pharmaceuticals, Inc. | 11-
Sep- 03 |
17-
Feb -05 |
11-
Feb -10 |
| US20100035
234 |
VACCINE ASSAYS | Novartis AG | 19-
May- 08 |
19-
May -09 |
11-
Feb -10 |
| US20100035
232 |
TARGETED WHOLE GENOME AMPLIFICATION METHOD FOR IDENTIFICATION OF PATHOGENS | ECKER DAVID J | 14-
Sep- 06 |
14-
Sep -07 |
11-
Feb -10 |
| US20100035
227 |
Compositions for use in identification of alphaviruses | ISIS Pharmaceuticals, Inc. | 3-
Mar- 04 |
2-
Mar -05 |
11-
Feb -10 |
| US20100034
852 |
METHODS AND COMPOSITIONS FOR PREDICTING AND TREATING DRUG RESISTANT STRAINS OF INFLUENZA VIRUS | NIMAN HENRY L | 25-
Jan- 07 |
24-
Jan -08 |
11-
Feb -10 |
| US20100029
732 |
Combinations of Beta-2-Adrenoceptor Agonistic Benzothiazolone | ASTRAZENECA AB | 8-
Feb- 07 |
6-
Feb -08 |
4-
Feb -10 |
| US20100029
722 |
ORGANIC COMPOUNDS | DALES NATALIE | 20-
Dec- 06 |
19-
Dec -07 |
4-
Feb -10 |
| US20100029
718 |
ORGANIC COMPOUNDS | Novartis AG | 22-
Sep- 06 |
19-
Sep -07 |
4-
Feb -10 |
| US20100029
713 |
QUINICLIDINE DERIVATIVES OF (HETERO)
ARYLCYCLOHEPTANECARBOXYLIC ACID AS MUSCARINIC RECEPTOR ANTAGONISTS |
ASTRAZENECA AB | 14-
Nov- 06 |
13-
Nov -07 |
4-
Feb -10 |
| US20100029
585 |
TOLL-LIKE RECEPTOR AGONIST FORMULATIONS AND THEIR USE | DIETSCH GREGORY | 1-
Aug- 08 |
31-
Jul- 09 |
4-
Feb -10 |
| US20100029
557 |
ALPHA-LACTALBUMIN COMPOSITION | GULDMANN MARIANNE | 17-
Nov- 06 |
15-
Nov -07 |
4-
Feb -10 |
| US20100029
552 |
Peptide inhibitors of c-jun dimerization and uses thereof | Phylogica Limited | 20-
Aug- 04 |
22-
Aug -05 |
4-
Feb -10 |
| US20100028
853 |
OPTICAL DETERMINATION OF LIVING VS. NON LIVING CELLS | Not Available | 25-
Oct- 05 |
2-
Nov -06 |
4-
Feb -10 |
| US20100028
381 |
FORMULATION FOR DELIVERY OF IMMUNE RESPONSE MODIFIERS | 3M INNOVATIVE PROPERTIES COMPANY | 19-
Jun- 06 |
18-
Jun -07 |
4-
Feb -10 |
| US20100028
369 |
Hazardous substance removing material and method for removing hazardous substance | FUJIFILM Corporation | 29-
Jul-08 |
28-
Jul- 09 |
4-
Feb -10 |
| US20100022
491 |
4-HYDROXY-2-OXO-2,3-DIHYDRO-1,3-BENZOTHIAZOL-7YL COMPOUNDS FOR MODULATION OF B2-ADRENORECEPTOR ACTIVITY | ASTRAZENECA AB | 20-
Dec- 06 |
19-
Dec -07 |
28-
Jan- 10 |
| US20100021
987 |
Compositions, Methods, and Kits for Enhancing Protein Expression | LIFESENSORS, INC. | 30-
Dec- 04 |
30-
Dec -05 |
28-
Jan- 10 |
| US20100021
907 |
METHOD OF DETECTING A PLURALITY OF NUCLEIC ACIDS | KABUSHIKI KAISHA TOSHIBA | 3-Jul-
08 |
12-
Jun -09 |
28-
Jan- 10 |
| US20100021
895 |
Vectors for Inducing Homozygous Mutations and Methods of Using Same | RULEY H EARL | 12-
Jul-06 |
12-
Jul- 07 |
28-
Jan- 10 |
| US20100021
470 |
MONOCLONAL ANTIBODY PRODUCTION BY EBV TRANSFORMATION OF B CELLS | INSTITUTE FOR RESEARCH IN BIOMEDICINE | 26-
Feb- 03 |
26-
Feb -04 |
28-
Jan- 10 |
| US20100016
388 |
Salts of a Selective Beta-2 Andrenoceptor Agonist | Not Available | 1-
Mar- 07 |
29-
Feb -08 |
21-
Jan- 10 |
| US20100016
359 |
METHODS FOR CONTROLLING SR PROTEIN PHOSPHORYLATION, AND ANTIVIRAL AGENTS WHOSE ACTIVE INGREDIENTS COMPRISE AGENTS THAT CONTROL SR PROTEIN ACTIVITY | Masatoshi Hagiwara | 26-
Dec- 03 |
29-
Jun -09 |
21-
Jan- 10 |
| US20100015
633 |
METHODS AND KIT FOR ANALYTE DETECTION | GENERAL ELECTRIC COMPANY | 18-
Jul-08 |
18-
Jul- 08 |
21-
Jan- 10 |
| US20100015
607 |
NANOREPORTERS AND METHODS OF MANUFACTURING AND USE THEREOF | INSTITUTE FOR SYSTEMS BIOLOGY | 23-
Dec- 05 |
22-
Dec -06 |
21-
Jan- 10 |
| US20100015
594 |
HUMAN PAPILLOMA VIRUS (HPV) DETECTION USING NUCLEIC ACID PROBES, MICROBEADS AND FLUORESCENT-ACTIVATED CELL SORTER (FACS) | GOULD TOBY | 10-
Dec- 04 |
9-
Dec -05 |
21-
Jan- 10 |
| US20100015
283 |
Method of Preparing Powder Kimchi and Kimchi Composition Using the Same | Not Available | 26-
Jun- 06 |
26-
Jun -07 |
21-
Jan- 10 |
| US20100015
211 |
Combination Approaches For Generating Immune Responses | BARNETT SUSAN W | 1-
Nov- 04 |
1-
Nov -05 |
21-
Jan- 10 |
| US20100015
173 |
COILED-COIL LIPOPEPTIDE HELICAL BUNDLES AND SYNTHETIC VIRUS-LIKE PARTICLES | UNIVERSITA/„T ZA/”RICH PROREKTORAT FORSCHUNG | 9-
Dec- 06 |
6-
Dec -07 |
21-
Jan- 10 |
| US20100010
217 |
METHODS FOR THE PREPARATION OF IMIDAZOLE-CONTAINING COMPOUNDS | LAN JIONG | 23-
Mar- 06 |
23-
Mar -07 |
14-
Jan- 10 |
| US20100010
199 |
MAKING INFLUENZA VIRUS VACCINES WITHOUT USING EGGS | NOVARTIS AG | 11-
Sep- 06 |
11-
Sep -07 |
14-
Jan- 10 |
| US20100010
080 |
USE OF TYLVALOSIN AS ANTIVIRAL AGENT | CAMBRIDGE UNIVERSITY TECHNICAL SERVICES | 13-
Jul-06 |
13-
Jul- 07 |
14-
Jan- 10 |
| US20100009
343 |
COMPOSITIONS AND METHOD FOR RAPID, REAL-TIME DETECTION OF INFLUENZA A VIRUS (H1N1) SWINE 2009 | Longhorn Vaccines & Diagnostics, LLC | 12-
Sep- 06 |
28-
Jul- 09 |
14-
Jan- 10 |
| US20100004
304 |
METHODS AND COMPOSITIONS FOR THE TREATMENT OF MALIGNANT MELANOMA, BREAST, PROSTATE, COLON, PAPILLARY THYROID AND PANCREATIC CANCER | GIULLANT CESIDIO | 16-
Mar- 04 |
1-
Oct -08 |
7-
Jan- 10 |
| US20100003
723 |
RNA-DEPENDENT DNA POLYMERASE FROM GEOBACILLUS STEAROTHERMOPHILUS | LAMPSON BERT C | 10-
Mar- 04 |
10-
Mar -04 |
7-
Jan- 10 |
| US20100003
277 |
VACCINES AND IMMUNOTHERAPEUTICS USING CODON OPTIMIZED IL-15 AND METHODS FOR USING THE SAME | KUTZLER MICHELE | 13-
Jan- 06 |
12-
Jan -07 |
7-
Jan- 10 |
| US20100003
269 |
METHODS AND USES OF CAULIFLOWER AND COLLARD FOR RECOMBINANT PROTEIN PRODUCTION | THOMAS JEFFERSON UNIVERSITY | 11-
Jul-06 |
28-
Jun -07 |
7-
Jan- 10 |
| US20090318
435 |
PYRAZOLOPYRIDINES AND ANALOGS THEREOF | BONK JASON D | 3-
Oct- 03 |
8-
Jun -09 |
24-
Dec -09 |
| US20090318
337 |
Compositions and methods for activating innate and allergic immunity | ID Biomedical Corporation of Quebec | 22-
Oct- 03 |
22-
Oct -04 |
24-
Dec -09 |
| US20090317
421 |
COMPOSITIONS AND METHODS RELATED TO STAPHYLOCOCCAL BACTERIUM PROTEINS | BURTS MONICA | 18-
Jan- 06 |
18-
Jan -07 |
24-
Dec -09 |
| US20090317
417 |
Modified AAV Vectors Having Reduced Capsid Immunogenicity and Use Thereof | The Trustees of the University of Pennsylvania | 28-
Apr- 06 |
27-
Apr -07 |
24-
Dec -09 |
| US20090312
285 |
BIOLOGICAL SPECIMEN COLLECTION AND TRANSPORT SYSTEM AND METHODS OF USE | Longhorn Vaccines & Diagnostics, LLC | 1-
Oct- 07 |
1-
Oct -08 |
17-
Dec -09 |
| US20090311
683 |
COMPOSITIONS FOR THE USE IN IDENTIFICATION OF FUNGI | IBIS BIOSCIENCES, INC. | 6-
Apr- 06 |
6-
Apr -07 |
17-
Dec -09 |
| US20090311
334 |
MUCOSAL IMMUNOGENIC SUBSTANCES COMPRISING A POLYINOSINIC ACID – POLYCYTIDILIC ACID BASED ADJUVANT | LI LIE TAO VICTOR | 13-
Jan- 06 |
27-
Jun -06 |
17-
Dec -09 |
| US20090311
288 |
IMIDAZOQUINOXALINE COMPOUNDS AS IMMUNOMODULATORS | Novartis AG | 23-
Mar- 06 |
23-
Mar -07 |
17-
Dec -09 |
| US20090306
347 |
ANTIBODY PRODUCED USING OSTRICH AND METHOD FOR PRODUCTION THEREOF | JAPAN SCIENCE AND TECHNOLOGY AGENCY | 29-
Aug- 05 |
29-
Aug -06 |
10-
Dec -09 |
| US20090306
090 |
HETEROCYCLIC DERIVATIVES AND THEIR USE AS STEAROYL-COA DESATURASE INHIBITORS | Xenon Pharmaceuticals Inc. | 20-
Sep- 04 |
13-
Aug -09 |
10-
Dec -09 |
| US20090306
042 |
Novel Compounds 010 | CAGE PETER ALAN | 12-
Dec- 07 |
10-
Dec -08 |
10-
Dec -09 |
| US20090305
282 |
NOVEL HUMAN VIRUS CAUSING RESPIRATORY TRACT INFECTION AND USES THEREOF | CHAN KWOK HUNG | 21-
Jul-04 |
1-
Jun -09 |
10-
Dec -09 |
| US20090304
743 |
Composition and Methods for Immunisation Using CD1D Ligands | GALLI GRAZIA | 15-
Mar- 06 |
15-
Mar -07 |
10-
Dec -09 |
| US20090304
742 |
INFLUENZA VACCINES WITH REDUCED AMOUNT OF EMULSION ADJUVANT | Novartis Vaccines and Diagnostics SRL | 4-
Nov- 05 |
6-
Nov -06 |
10-
Dec -09 |
| US20090304
739 |
INFLUENZA VACCINES INCLUDING COMBINATIONS OF PARTICULATE ADJUVANTS AND IMMUNOPOTENTIATORS | NOVARTIS VACCINES AND DIAGNOSTICS SRL | 4-
Nov- 05 |
6-
Nov -06 |
10-
Dec -09 |
| US20090304
729 |
CELL-DERIVED VIRAL VACCINES WITH LOW LEVELS OF RESIDUAL CELL DNA | NOVARTIS VACCINES AND DIAGNOSTICS GMBH & CO KG | 1-
Nov- 05 |
1-
Nov -06 |
10-
Dec -09 |
| US20090304
683 |
Soluble Fragments of The Sars-Cov Spike Glycoprotein | DIMITROV DIMITER S | 19-
Jan- 07 |
19-
Jan -07 |
10-
Dec -09 |
| US20090304
681 |
Methods for the treatment and prevention of infection using anti- selectin agents | Not Available | 29- Jul-04 | 27-
Jul- 05 |
10-
Dec -09 |
| US20090304
630 |
Treating severe and acute viral infections | HEMISPHERx BIOPHARMA | 16-
May- 03 |
2-
Aug -07 |
10-
Dec -09 |
| US20090300
778 |
Neutral Sphingomyelinase-E and Its Use | HOPPE UTA | 8-
Feb- 06 |
8-
Feb -07 |
3-
Dec -09 |
| US20090299
250 |
ANTIVIRAL FILTER AND ITS USE IN AN AIR PURIFIER, AIR
CONDITIONER OR AIR HUMIDIFIER |
Université De Rouen | 19-
Jul-06 |
19-
Jul- 07 |
3-
Dec -09 |
| US20090298
914 |
RNA Interference Mediated Inhibition of Severe Acute Respiratory Syndrome (SARS) Virus Gene Expression Using Short Interfering Nucleic Acid (siNA) | Sirna Therapeutics, Inc. | 15-
Apr- 03 |
17-
Nov -08 |
3-
Dec -09 |
| US20090298
821 |
HYDROXY AND ALKOXY SUBSTITUTED IH-
IMIDAZONAPHTHYRIDINES AND METHODS |
Pfizer Inc. | 15-
Mar- 06 |
14-
Mar -07 |
3-
Dec -09 |
| US20090298
807 |
Compounds | ALCARAZ LILIAN | 6-
Feb- 08 |
4-
Feb -09 |
3-
Dec -09 |
| US20090297
555 |
RECOMBINANT HUMAN CYTOMEGALOVIRUS AND VACCINES COMPRISING HETEROLOGOUS ANTIGENS | MEDIMMUNE, LLC | 25-
Jun- 04 |
30-
Jun -09 |
3-
Dec -09 |
| US20090286
835 |
NOVEL COMPOUNDS | AstraZeneca AB | 4-
Oct- 07 |
3-
Oct -08 |
19-
Nov -09 |
| US20090286
813 |
Thioxanthine Derivatives and Their Use as Inhibitors of MPO | ASTRAZENECA AB | 13-
Apr- 06 |
12-
Apr -07 |
19-
Nov -09 |
| US20090286
726 |
USE OF THE LONG PENTRAXIN PTX3 FOR THE PREVENTION OR TREATMENT OF VIRAL DISEASES | TECNOGEN S.P.A. | 10-
Mar- 06 |
28-
Feb -07 |
19-
Nov -09 |
| US20090286
222 |
Mixed Cell Diagnostic Systems For Detection Of Respiratory, Herpes And Enteric Viruses | GOODRUM PATRICIA GAIL RAY | 24-
Apr- 98 |
16-
Oct -08 |
19-
Nov -09 |
| US20090285
921 |
PLANT EXTRACT AND ITS THERAPEUTIC USE | Veritron Limited | 16-
May- 08 |
1-
Dec -08 |
19-
Nov -09 |
| US20090285
901 |
Polyamino acid for use as adjuvant | AKASHI MITSURU | 20-
Apr- 05 |
27-
Jul- 09 |
19-
Nov -09 |
| US20090285
854 |
FROZEN STOCKPILING OF INFLUENZA VACCINES | Novartis AG | 20-
Jul-06 |
20-
Jul- 07 |
19-
Nov -09 |
| US20090281
042 |
COMPOSITIONS AND METHODS USING SAME FOR THE DETECTION OF VIRUSES | ARAD DORIT | 8-
Sep- 05 |
10-
Sep -06 |
12-
Nov -09 |
| US20090281
041 |
ANTIVIRAL CELL-PENETRATING PEPTIDES | CURRELI FRANCESCA | 6-
May- 08 |
5-
May -09 |
12-
Nov -09 |
| US20090280
507 |
METHOD FOR MEASUREMENT OF SARS VIRUS NUCLEOCAPSID PROTEIN, REAGENT KIT FOR THE MEASUREMENT, TEST DEVICE,
MONOCLONAL ANTIBODY DIRECTED AGAINST SARS VIRUS NUCLEOCAPSID PROTEIN, AND HYBRIDOMA CAPABLE OF PRODUCING THE MONOCLONAL ANTIBODY |
SYSMEX CORPORATION | 11-
Oct- 05 |
11-
Oct -06 |
12-
Nov -09 |
| US20090275
666 |
CONDENSATION PRODUCTS, METHOD FOR THEIR PRODUCTION
AND USE THEREOF IN MEDICAMENTS, AS DISINFECTANTS OR AS A TANNIN |
BASF SE | 17-
May- 06 |
28-
Feb -07 |
5-
Nov -09 |
| US20090275
583 |
ANTIVIRAL COMPOUNDS AND USE THEREOF | Myriad Genetics, Incorporated | 13-
Oct- 06 |
13-
Apr -09 |
5-
Nov -09 |
| US20090275
508 |
USE OF THYMOSIN ALPHA 1, ALONE OR IN COMBINATION WITH PTX3 OR GANCICLOVIR, FOR THE TREATMENT OF CYTOMEGALOVIRUS INFECTION | SIGMA-TAU INDUSTRIE FARMACEUTICHE RIUNITE S.P.A. | 2-
May- 06 |
12-
Apr -07 |
5-
Nov -09 |
| US20090275
016 |
ARRAYED DETECTOR SYSTEM FOR MEASUREMENT OF INFLUENZA IMMUNE RESPONSE | UNIVERSITY OF ROCHESTER | 2-
May- 08 |
1-
May -09 |
5-
Nov -09 |
| US20090270
443 |
1-AMINO IMIDAZO-CONTAINING COMPOUNDS AND METHODS | Not Available | 2-
Sep- 04 |
2-
Sep -05 |
29-
Oct- 09 |
| US20090270
431 |
Cyclopentenol Nucleoside Compounds Intermediates for their Synthesis and Methods of Treating Viral Infections | THE UNIVERSITY OF GEORGIA RESEARCH FOUNDATION | 19-
Oct- 05 |
19-
Oct -06 |
29-
Oct- 09 |
| US20090269
367 |
METHODS AND COMPOUNDS FOR MITIGATING PATHOGENIC OUTBREAKS USING REPLIKIN COUNT CYCLES | BOGOCH ELENORE S | 23-
Apr- 08 |
23-
Apr -09 |
29-
Oct- 09 |
| US20090264
653 |
USEFUL INDOLE COMPOUNDS | BARDEN TIMOTHY | 16-
Dec- 05 |
18-
Dec -06 |
22-
Oct- 09 |
| US20090264
509 |
Adenoviral vector-based foot-and-mouth disease vaccine | GENVEC, INC. | 10-
Nov- 05 |
8-
May -08 |
22-
Oct- 09 |
| US20090264
444 |
Organic compounds | CHOWDHURY SULTAN | 20-
Feb- 08 |
20-
Feb -09 |
22-
Oct- 09 |
| US20090264
362 |
Influenza virus inhibiting peptides | The Administrators of the Tulane Educational Fund | 4-
Nov- 03 |
17-
Feb -09 |
22-
Oct- 09 |
| US20090264
307 |
ARRAY-BASED POLYMORPHISM MAPPING AT SINGLE NUCLEOTIDE RESOLUTION | The Trustees of Princeton University | 13-
Jan- 06 |
12-
Jan -07 |
22-
Oct- 09 |
| US20090264
300 |
ENZYMATIC ENCODING METHODS FOR EFFICIENT SYNTHESIS OF LARGE LIBRARIES | Nuevolution A/S | 1-
Dec- 05 |
1-
Dec -06 |
22-
Oct- 09 |
| US20090263
352 |
METHODS FOR TREATING VIRAL INFECTION USING IL-28 AND IL- 29 CYSTEINE MUTANTS | ZymoGenetics, LLC | 2-
Apr- 04 |
21-
May -09 |
22-
Oct- 09 |
| US20090258
440 |
SURFACE FOR LABEL INDEPENDENT DETECTION AND METHOD THEREOF | BUNCH THOMAS A | 10-
Apr- 08 |
3-
Apr -09 |
15-
Oct- 09 |
| US20090258
340 |
Assay for SARS coronavirus by amplification and detection of the replicase sequence | Becton, Dickinson and Company | 12-
Sep- 03 |
3-
Feb -09 |
15-
Oct- 09 |
| US20090258
074 |
MIXTURES OF TANNINS, THEIR PRODUCTION AND USE IN MEDICAMENTS OR AS DISINFECTANTS | BASF SE | 17-
May- 06 |
28-
Feb -07 |
15-
Oct- 09 |
| US20090258
011 |
Antibodies against west nile virus and therapeutic and prophylactic uses thereof | Washington University | 21-
Jun- 04 |
6-
Apr -09 |
15-
Oct- 09 |
| US20090257
950 |
Membrane Scaffold Proteins | THE BOARD OF TRUSTEES OF THE UNIVERSITY OF
ILLINOIS |
20-
Nov- 00 |
10-
Oct -07 |
15-
Oct- 09 |
| US20090253
714 |
Methods of reducing risk of infection from pathogens | Not Available | 20-
Aug- 03 |
18-
Aug -04 |
8-
Oct- 09 |
| US20090253
695 |
Hydroxyalkyl Substituted Imidazonaphthyridines | Coley Pharmaceutical Group, Inc, | 23-
Feb- 05 |
22-
Feb -06 |
8-
Oct- 09 |
| US20090252
775 |
VIRUCIDAL DISINFECTANT | B. BRAUN MEDICAL AG | 28-
Jan- 05 |
28-
May -09 |
8-
Oct- 09 |
| US20090252
646 |
STERILIZATION METHODS AND SYSTEMS FOR GAMING EQUIPMENT | Invention Factory, LLC | 8-
Jan- 07 |
28-
May -09 |
8-
Oct- 09 |
| US20090247
614 |
Folate Conjugates | ALNYLAM PHARMACEUTICALS, INC. | 4-
Dec- 07 |
4-
Dec -08 |
1-
Oct- 09 |
| US20090247
608 |
Targeting Lipids | ALNYLAM PHARMACEUTICALS, INC. | 4-
Dec- 07 |
4-
Dec -08 |
1-
Oct- 09 |
| US20090239
848 |
NOVEL PIPERAZINE DERIVATIVES AS INHIBITORS OF STEAROYL- CoA DESATURASE | FOREST LABORATORIES HOLDINGS LIMITED | 20-
Mar- 08 |
20-
Mar -09 |
24-
Sep -09 |
| US20090239
830 |
T reatment of viral infections by modulation of host cell metabolic pathways | BENNETT BRYSON | 1-
Jun- 07 |
2-
Jun -08 |
24-
Sep -09 |
| US20090239
824 |
HYDROLYTICALLY-RESISTANT BORON-CONTAINING THERAPEUTICS AND METHODS OF USE | Anacor Pharmaceuticals, Inc. | 16-
Jun- 03 |
13-
Nov -08 |
24-
Sep -09 |
| US20090239
814 |
Carbohydrate Conjugates as Delivery Agents for Oligonucleotides | ALNYLAM PHARMACEUTICALS, INC. | 4-
Dec- 07 |
4-
Dec -08 |
24-
Sep -09 |
| US20090239
810 |
NOVEL PIPERAZINE DERIVATIVES AS INHIBITORS OF STEAROYL- CoA DESATURASE | Forest Laboratories Holdings Limited | 20-
Mar- 08 |
20-
Mar -09 |
24-
Sep -09 |
| US20090235
625 |
FILTER AND ASSOCIATED METHOD | GENERAL ELECTRIC COMPANY | 28-
Sep- 07 |
28-
Sep -07 |
24-
Sep -09 |
| US20090233
965 |
Alkyl Esters Of Cyclic Amino Alcohols With Muscarinic M3 Receptor Antagonist Activity, Useful For Treating E.G. Chronic Bronchial Obstruction, Asthma And Overactive Bladder | ASTRAZENECA AB | 24-
Apr- 06 |
23-
Apr -07 |
17-
Sep -09 |
| US20090233
907 |
Pyridopyrimidine Derivatives and Their Use as PDE4 Inhibitors | ASTRAZENECA AB | 22-
Mar- 06 |
20-
Mar -07 |
17-
Sep -09 |
| US20090233
309 |
BIOLOGICAL SPECIMEN COLLECTION/TRANSPORT COMPOSITIONS AND METHODS | LONGHORN VACCINES & DIAGNOSTICS, LLC | 1-
Oct- 07 |
20-
Apr -09 |
17-
Sep -09 |
| US20090232
844 |
IMMUNOPOTENTIATING COMPOUNDS | SUTTON JAMES | 23-
Mar- 06 |
23-
Mar -07 |
17-
Sep -09 |
| US20090232
843 |
Identifying and predicting influenza variants and uses thereof | NIMAN HENRY L | 8-Jul-
05 |
4-
Jan -08 |
17-
Sep -09 |
| US20090232
808 |
MOLECULES AND CHIMERIC MOLECULES THEREOF | APOLLO LIFE SCIENCES LIMITED | 28-
Jan- 05 |
27-
Jan -06 |
17-
Sep -09 |
| US20090232
748 |
Virucidal activities of cetylpyridinium chloride | ViraTox, L.L.C. | 7-
Nov- 03 |
18-
Mar -09 |
17-
Sep -09 |
| US20090227
637 |
DIARYL UREAS FOR TREATING VIRUS INFECTIONS | RIEDL BERND | 15-
Dec- 05 |
6-
Dec -06 |
10-
Sep -09 |
| US20090227
541 |
BORON-CONTAINING SMALL MOLECULES | Anacor Pharmaceuticals, Inc. | 20-
Jun- 07 |
19-
Jun -08 |
10-
Sep -09 |
| US20090227
030 |
Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
31-
Oct -07 |
10-
Sep -09 |
| US20090227
005 |
Methods for pathogen detection | Searete LLC, a limited liability corporation of the State of Delaware | 27-
Mar- 07 |
28-
Sep -07 |
10-
Sep -09 |
| US20090226
885 |
Nucleic Acid Sequences That Can Be Used As Primers And Probes In The Amplification And Detection Of Sars Coronavirus | OVERDIJK MARLIEKE | 10-
Jun- 03 |
8-
Mar -04 |
10-
Sep -09 |
| US20090222
936 |
Recombinant Expression of Multiprotein Complexes Using Polygenes | BERGER IMRE | 8-
Nov- 05 |
6-
Nov -06 |
3-
Sep -09 |
| US20090221
653 |
7-(2-amino-1-hydroxy-ethyl)-4-hydroxybenzothiazol-2(3H)-one- derivatives as beta2 adrenoreceptor agonists | AstraZeneca AB | 29-
Aug- 05 |
28-
Aug -06 |
3-
Sep -09 |
| US20090221
640 |
Novel Crystal Modifications | ASTRAZENECA AB | 16-
Mar- 06 |
15-
Mar -07 |
3-
Sep -09 |
| US20090221
631 |
IMIDAZOPYRIDINONES | Not Available | 3-
Aug- 07 |
15-
May -09 |
3-
Sep -09 |
| US20090221
624 |
4-AMINOQUINOLINE COMPOUNDS FOR TREATING VIRUS-RELATED CONDITIONS | BUSCHER BENJAMIN A | 6-
May- 05 |
4-
May -06 |
3-
Sep -09 |
| US20090221
583 |
NOVEL PIPERAZINE DERIVATIVES AS INHIBITORS OF STEAROYL- CoA DESATURASE | AURIGENE DISCOVERY TECHNOLOGIES LIMITED | 21-
Jun- 07 |
23-
Jun -08 |
3-
Sep -09 |
| US20090221
556 |
HYDROXY AND ALKOXY SUBSTITUTED 1H-IMIDAZOQUINOLINES AND METHODS | Pfizer Inc. | 4-
Nov- 05 |
3-
Nov -06 |
3-
Sep -09 |
| US20090221
551 |
SUBSTITUTED FUSED[1,2] IMIDAZO[4,5C] RING COMPOUNDS AND METHODS | Pfizer Inc. | 15-
Mar- 06 |
14-
Mar -07 |
3-
Sep -09 |
| US20090221
487 |
POLYETHLENE GLYCOL MODIFICATIONS OF THYMOSIN ALPHA-1 | Institute of Pharmacology and Toxicology Academy of Military Medical Sciences P.L.A. China | 10-
Nov- 05 |
11-
Nov -06 |
3-
Sep -09 |
| US20090220
941 |
COMPOSITIONS FOR- DETECTING OF INFLUENZA VIRUSES AND KITS AND METHODS USING SAME | MND DIAGNOSTIC LTD | 25-
Oct- 05 |
25-
Oct -06 |
3-
Sep -09 |
| US20090220
937 |
Compositions for Use in Identification of Adventitious Viruses | IBIS BIOSCIENCES, INC. | 3-
Mar- 05 |
3-
Mar -06 |
3-
Sep -09 |
| US20090220
547 |
Reducing interference between oil-containing adjuvants and surfactant-containing antigens | NOVARTIS VACCINES AND DIAGNOSTICS SRL | 2-
Aug- 05 |
2-
Aug -06 |
3-
Sep -09 |
| US20090220
546 |
Adjuvanted influenza vaccines for pediatric use | GROTH NICOLA | 22-
Feb- 08 |
20-
Feb -09 |
3-
Sep -09 |
| US20090220
545 |
Adjuvant-Sparing Multi-Dose Influenza Vaccination Regimen | NOVARTIS AG | 15-
Jun- 06 |
15-
Jun -07 |
3-
Sep -09 |
| US20090220
544 |
ADJUVANTED VACCINES WITH NON-VIRION ANTIGENS PREPARED FROM INFLUENZA VIRUSES GROWN IN CELL CULTURE | NOVARTIS VACCINES AND DIAGNOSTICS SRL | 4-
Nov- 05 |
6-
Nov -06 |
3-
Sep -09 |
| US20090220
541 |
EMULSIONS WITH FREE AQUEOUS-PHASE SURFACTANT FOR ADJUVANTING SPLIT INFLUENZA VACCINES | NOVARTIS VACCINES AND DIAGNOSTICS SRL | 4-
Nov- 05 |
6-
Nov -06 |
3-
Sep -09 |
| US20090220
530 |
DEFECTIVE RIBOSOMAL PRODUCTS IN BLEBS (DRIBBLES) AND METHODS OF USE TO STIMULATE AN IMMUNE RESPONSE | HU HONG-MING | 29-
Jul-05 |
27-
Jul- 06 |
3-
Sep -09 |
| US20090220
514 |
ADAM10 and its Uses Related to Infection | HODGE THOMAS | 13-
Jan- 06 |
16-
Jan -07 |
3-
Sep -09 |
| US20090220
456 |
USES OF INTERFERONS WITH ALTERED SPATIAL STRUCTURE | Not Available | 28-
Aug- 03 |
11-
Feb -09 |
3-
Sep -09 |
| US20090216
860 |
SYSTEM AND METHOD FOR DETECTING, COLLECTING, ANALYZING, AND COMMUNICATING EVENT RELATED INFORMATION | Georgetown University | 25-
Feb- 08 |
19-
Nov -08 |
27-
Aug -09 |
| US20090216
747 |
System and method for detecting, collecting, analyzing, and communicating event-related information | Georgetown University- OTC | 25-
Feb- 08 |
28-
Aug -08 |
27-
Aug -09 |
| US20090215
786 |
NOVEL CYSTEINE PROTEASE INHIBITORS AND THEIR THERAPEUTIC APPLICATIONS | BOISSY GUILLAUME | 5-
Aug- 05 |
26-
Jul- 06 |
27-
Aug -09 |
| US20090214
663 |
Virus coated nanoparticles and uses thereof | ALBRECHT THOMAS B | 26-
Sep- 06 |
26-
Mar -09 |
27-
Aug -09 |
| US20090214
590 |
Virus Vaccines Comprising Envelope-Bound Immunomodulatory Proteins and Methods of Use Thereof | Wayne State University | 8-Jul-
05 |
10-
Jul- 06 |
27-
Aug -09 |
| US20090214
587 |
Recombinant virus and use thereof | Post Genome Institute Co., Ltd. | 8-
Oct- 04 |
11-
Oct -05 |
27-
Aug -09 |
| US20090214
537 |
Serum Resistance Factors of Gram Positive Bacteria | NOVARTIS VACCINES AND DIAGNOSTICS, INC. | 13-
May- 05 |
12-
May -06 |
27-
Aug -09 |
| US20090214
533 |
METHODS FOR CONVERTING OR INDUCING PROTECTIVE IMMUNITY | THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK | 17-
Aug- 06 |
17-
Feb -09 |
27-
Aug -09 |
| US20090214
510 |
Broad Spectrum Antiviral Compositions | Government of the US as represented by the Secretary, Department of Health and Human Services | 21-
Feb- 06 |
21-
Feb -07 |
27-
Aug -09 |
| US20090214
444 |
POLY AROMATIC SODIUM CHANNEL BLOCKERS | PARION SCIENCES, Inc. | 26-
Feb- 08 |
26-
Feb -09 |
27-
Aug -09 |
| US20090209
640 |
Prevention of and countermeasures against viral infection | CHOKO CO., LTD. | 20-
Nov- 03 |
15-
Apr -09 |
20-
Aug -09 |
| US20090209
555 |
2-PYRAZINONE DERIVATIVES FOR THE TREATMENT OF DISEASE OR CONDITION IN WHICH INHIBITION OF NEUTROPHIL ELASTASE ACTIVITY IS BENEFICIAL | HANSEN PETER | 8-
May- 06 |
7-
May -07 |
20-
Aug -09 |
| US20090209
524 |
Novel Compounds | AstraZeneca AB | 22-
Nov- 07 |
20-
Nov -08 |
20-
Aug -09 |
| US20090209
505 |
PRODRUGS OF HETEROARYL COMPOUNDS | Koronis Pharmaceuticals, Incorporated | 20-
Jun- 03 |
7-
Apr -09 |
20-
Aug -09 |
| US20090209
458 |
Cell-penetrating socs polypeptides that inhibit cytokine-induced signaling | VANDERBILT UNIVERSITY | 4-
Mar- 04 |
4-
Mar -05 |
20-
Aug -09 |
| US20090208
531 |
ANTIVIRAL AGENTS AND VACCINES AGAINST INFLUENZA | National Institutes of Health Office of Technology | 16-
Feb- 06 |
16-
Feb -07 |
20-
Aug -09 |
| US20090205
116 |
ARTICLE, LAMINATE AND ASSOCIATED METHODS | GENERAL ELECTRIC COMPANY | 30-
Sep- 05 |
5-
Dec -08 |
20-
Aug -09 |
| US20090203
753 |
7-(2-amino-1-hydroxy-ethyl)-4-hydroxybenzothiazol-2(3H)-one- derivatives as beta2 adrenoreceptor agonists | AstraZeneca AB | 29-
Aug- 05 |
28-
Aug -06 |
13-
Aug -09 |
| US20090203
675 |
Sulfonyl Semicarbazides, Semicarbazides and Ureas, Pharmaceutical Compositions Thereof, and Methods for Treating Hemorrhagic Fever Viruses, Including Infections Associated with Arena Viruses | SIGA TECHNOLOGIES, INC. | 6-
Dec- 04 |
6-
Dec -05 |
13-
Aug -09 |
| US20090203
112 |
VERO CELL LINE WHICH IS ADAPTED TO GROW IN SUSPENSION | Ricardo Kratje | 4-
Oct- 05 |
3-
Oct -06 |
13-
Aug -09 |
| US20090202
995 |
MOLECULAR CARDIOTOXICOLOGY MODELING | JOHNSON KORY R | 26-
Aug- 05 |
28-
Aug -06 |
13-
Aug -09 |
| US20090202
590 |
INFLUENZA VACCINES EXTEMPORANEOUSLY ADSORBED TO ALUMINIUM ADJUVANTS | NOVARTIS VACCINES AND DIGNOSTICS SRL | 4-
Nov- 05 |
6-
Nov -06 |
13-
Aug -09 |
| US20090197
914 |
Piperidine Derivatives, Their Process for Preparation, Their Use as Therapeutic Agents and Pharmaceutical Compositions Containing Them | CAGE PETER | 7-
Mar- 06 |
6-
Mar -07 |
6-
Aug -09 |
| US20090197
894 |
NICOTINAMIDE DERIVATIVES AND THEIR USE AS THERAPEUTIC AGENTS | XENON PHARMACEUTICALS INC. | 21-
Dec- 01 |
14-
Apr -09 |
6-
Aug -09 |
| US20090197
890 |
PYRIDAZINE DERIVATIVES AND THEIR USE AS THERAPEUTIC
AGENTS |
XENON PHARMACEUTICALS INC. | 30-
Jul-03 |
20-
Feb -09 |
6-
Aug -09 |
| US20090197
338 |
Method of Increasing the Function of an AAV Vector | The Trustees of teh University of Pennsylvania | 7-
Apr- 05 |
7-
Apr -06 |
6-
Aug -09 |
| US20090196
921 |
Compositions Methods and Kits For Enhancing Immune Response To A Respiratory Condition | The Procter & Gamble Company | 6-
Feb- 08 |
6-
Feb -09 |
6-
Aug -09 |
| US20090192
505 |
Method for cryospray ablation | Reset Medical, Inc. | 5-
Dec- 07 |
5-
Dec -08 |
30-
Jul- 09 |
| US20090192
163 |
Novel Compounds | ASTRAZENECA AB | 6-
Oct- 05 |
5-
Oct -06 |
30-
Jul- 09 |
| US20090192
153 |
NOVEL ADENINE COMPOUND | AstraZeneca Aktiebolag A corporation of Sweden | 22-
Sep- 05 |
21-
Sep -06 |
30-
Jul- 09 |
| US20090192
099 |
PRODRUGS OF HETEROARYL COMPOUNDS | Koronis Pharmaceuticals, Incorporated | 20-
Jun- 03 |
30-
Mar -09 |
30-
Jul- 09 |
| US20090192
090 |
PEPTIDES AND PEPTIDOMIMETICS HAVING IMMUNE-MODULATING, ANTI-INFLAMMATORY, AND ANTI-VIRAL ACTIVITY | TAKEDA PHARMACEUTICAL COMPANY LIMITED | 25-
Jun- 03 |
31-
Dec -08 |
30-
Jul- 09 |
| US20090192
081 |
Casein derived peptides and uses thereof | SIDELMAN ZVI | 1-
Mar- 00 |
31-
Oct -07 |
30-
Jul- 09 |
| US20090191
229 |
ADJUVANCY AND IMMUNE POTENTIATING PROPERTIES OF NATURAL PRODUCTS OF ONCHOCERCA VOLVULUS | NEW YORK BLOOD CENTER | 15-
Jun- 04 |
23-
Mar -09 |
30-
Jul- 09 |
| US20090186
056 |
COMPOSITIONS AND METHODS FOR PREVENTING INFECTION | LA JOLLA BIOSCIENCES LLC | 8-
Aug- 02 |
19-
Mar -09 |
23-
Jul- 09 |
| US20090186
041 |
ANTI-TSG101 ANTIBODIES AND THEIR USES FOR TREATMENT OF VIRAL INFECTIONS | Functional Genetics, Inc. | 15-
Nov- 06 |
18-
Apr -08 |
23-
Jul- 09 |
| US20090186
025 |
Fusion Protein Comprising an Fc Receptor Binding Polypeptide and an Antigenic Polypeptide for Mediating an Immune Response | ImmunoBiology Limited | 22-
Mar- 06 |
22-
Mar -07 |
23-
Jul- 09 |
| US20090186
014 |
METHOD FOR TREATMENT OF PANCREATITIS | Ore Pharmaceuticals Inc. | 10-
Oct- 07 |
9-
Oct -08 |
23-
Jul- 09 |
| US20090182
133 |
BENZOPYRANONE DERIVATIVES AND THEIR USE AS ANTI-VIRAL AGENTS | Shanghai Institute of Materia Medica Chinese Academy of Sciences | 29-
Dec- 05 |
15-
Dec -06 |
16-
Jul- 09 |
| US20090181
972 |
Novel Inhibitors of Cysteine Proteases, the Pharmaceutical Compositions Thereof and their Therapeutic Applications | BOISSY GUILLAUNE | 8-
Dec- 05 |
5-
Dec -06 |
16-
Jul- 09 |
| US20090181
361 |
Rapid test for detecting infection | KUMAR ARUN | 14-
Jan- 08 |
14-
Jan -08 |
16-
Jul- 09 |
| US20090176
821 |
Amide and Carbamate Derivatives of Alkyl Substituted N-[4-(4- Amino- 1H-Imidazo[4,5-C] Quinolin-1-
YL)Butyl]Methanesulfonamides and Methods |
COLEY PHARMACEUTICAL GROUP, INC. | 9-
Sep- 05 |
8-
Sep -06 |
9-
Jul- 09 |
| US20090176
815 |
Novel Tricyclic Spiropiperidine Compounds, Their Synthesis and Their Uses as Modulators of Chemokine Receptor Activity | ERIKSSON TOMAS | 19-
Jul-06 |
16-
Jan -09 |
9-
Jul- 09 |
| US20090176
699 |
Inhibitors Based on Fusion, Hr1 and Hr2 Sequences in Bacterial Adhesin | CHIRON SRL | 6-Jul-
04 |
6-
Jul- 05 |
9-
Jul- 09 |
| US20090175
948 |
AEROSOL METHOD FOR NANO SILVER-SILICA COMPOSITE ANTIMICROBIAL AGENT | BRINKER C JEFFREY | 30-
Nov- 07 |
26-
Nov -08 |
9-
Jul- 09 |
| US20090175
902 |
Immunogenic Substances Comprising A Polyinosinic Acid- Polycytidilic Acid Based Adjuvant | Not Available | 13-
Jan- 06 |
27-
Jun -06 |
9-
Jul- 09 |
| US20090170
717 |
RE-SEQUENCING PATHOGEN MICROARRAY | The Government of the United States of America, as represented by the Secretary of the Air Force | 2-Jul-
04 |
10-
Apr -08 |
2-
Jul- 09 |
| US20090169
636 |
Microparticles containing biodegradable polymer and cationic polysaccharide for use in immunogenic compositions | Not Available | 24-
Feb- 06 |
24-
Feb -07 |
2-
Jul- 09 |
| US20090163
533 |
1-Substituted Pyrazolo (3,4-C) Ring Compounds as Modulators of Cytokine Biosynthesis for the Treatment of Viral Infections and Neoplastic Diseases | COLEY PHARMACEUTICAL GROUP, INC. | 1-
Apr- 05 |
31-
Mar -06 |
25-
Jun- 09 |
| US20090163
518 |
Novel Compounds | BONNERT ROGER | 27-
May- 03 |
4-
Mar -09 |
25-
Jun- 09 |
| US20090162
831 |
HUMAN PARVOVIRUS | DELWART ERIC L | 24-
May- 04 |
24-
May -05 |
25-
Jun- 09 |
| US20090162
392 |
MUTANT FORMS OF STREPTOLYSIN O | NOVARTIS AG | 21-
Dec- 07 |
19-
Dec -08 |
25-
Jun- 09 |
| US20090162
365 |
Novel siRNAS and methods of use thereof | FEINSTEIN ELENA | 25-
Oct- 06 |
25-
Oct -07 |
25-
Jun- 09 |
| US20090162
320 |
GENE TRANSFER INTO AIRWAY EPITHELIAL STEM CELL BY USING LENTIVIRAL VECTOR PSEUDOTYPED WITH RNA VIRUS OR DNA
VIRUS SPIKE PROTEIN |
DNAVEC Corporation | 28-
Oct- 05 |
27-
Oct -06 |
25-
Jun- 09 |
| US20090155
564 |
ARTICLE AND ASSOCIATED METHOD | GENERAL ELECTRIC COMPANY | 28-
Sep- 07 |
28-
Sep -07 |
18-
Jun- 09 |
| US20090149
475 |
Thioxanthine Derivatives and Their Use as Inhibitors of MPO | ASTRAZENECA AB | 13-
Apr- 06 |
12-
Apr -07 |
11-
Jun- 09 |
| US20090149
448 |
Phenoxyacetic Acid Derivatives Useful for Treating Respiratory Diseases | ASTRAZENECA AB | 23-
Nov- 04 |
22-
Nov -05 |
11-
Jun- 09 |
| US20090149
429 |
ANTIVIRAL COMPOUNDS | Myriad Genetics, Incorporated | 22-
Jun- 05 |
22-
Jun -06 |
11-
Jun- 09 |
| US20090131
486 |
2-PYRIDONE DERIVATIVES FOR THE TREATMENT OF DISEASE OR CONDITION IN WHICH INHIBITION OF NEUTROPHIL ELASTASE ACTIVITY IS BENEFICIAL | HANSEN PETER | 8-
May- 06 |
7-
May -07 |
21-
May -09 |
| US20090131
459 |
2-Thioxanthine Derivatives Acting as MPO-Inhibitors | ASTRAZENECA AB | 5-
Jun- 06 |
4-
Jun -07 |
21-
May -09 |
| US20090131
328 |
FURIN INHIBITORS | SMITH JUDITH | 23-
Jul-04 |
22-
Jul- 05 |
21-
May -09 |
| US20090130
146 |
COMBINATION VACCINE | CHIRON BEHRING GMBH & CO. KG | 8-
Oct- 04 |
7-
Oct -05 |
21-
May -09 |
| US20090130
138 |
Antiviral and antibacterial activity from medicinal mushrooms | STAMETS PAUL EDWARD | 6-
Jan- 04 |
24-
Sep -08 |
21-
May -09 |
| US20090126
514 |
Devices for collection and preparation of biological agents | BELGRADER PHIL | 5-
Sep- 07 |
5-
Sep -07 |
21-
May -09 |
| US20090124
843 |
HAZARDOUS SUBSTANCE REMOVING METHOD, HAZARDOUS SUBSTANCE REMOVING MATERIAL USED THEREIN SUCH AS AIR FILTER, MASK, WIPE SHEET, AND THE LIKE, AND STORAGE METHOD THEREOF | Not Available | 28-
Mar- 03 |
21-
Nov -08 |
14-
May -09 |
| US20090124
652 |
Polymorphs of 1-(2-Methylpropyl)-1H-Imidazo[4,5- C][1,5]Naphthyridin-4-Amine Ethane-Sulfonate | Takeda Pharmaceutical Company Limited | 30-
Dec- 04 |
28-
Dec -05 |
14-
May -09 |
| US20090124
640 |
Pyrrolo[3,2-D]Pyrimidin-4-One Derivative as Myeloperoxidase Inhibitor | ASTRAZENECA AB | 5-
Jun- 06 |
4-
Jun -07 |
14-
May -09 |
| US20090124
611 |
Pyrazolopyridine-1,4-Diamines and Analogs Thereof | Coley Pharmaceutical Group, Inc. | 1-
Apr- 05 |
31-
Mar -06 |
14-
May -09 |
| US20090124
596 |
Chemical Compounds 637 | BONNERT ROGER VICTOR | 11-
Jan- 07 |
10-
Jan -08 |
14-
May -09 |
| US20090124
512 |
DNA ARRAY ANALYSIS AS A DIAGNOSTIC FOR CURRENT AND EMERGING STRAINS OF INFLUENZA | REGENTS OF THE UNIVERSITY OF COLORADO | 18-
Jan- 06 |
18-
Jan -07 |
14-
May -09 |
| US20090123
494 |
MOMLV-BASED PSEUDOVIRION PACKAGING CELL LINE | FLICK RAMON | 31-
Jul-07 |
31-
Jul- 08 |
14-
May -09 |
| US20090123
460 |
IMMUNOSTIMULATORY COMBINATIONS | 3M Innovative Properties Company | 30-
Dec- 02 |
17-
Mar -08 |
14-
May -09 |
| US20090123
417 |
RECOMBINANT SUPER-COMPOUND INTERFERON AND USES THEREOF | WEI GUANGWEN | 28-
Feb- 01 |
6-
Oct -08 |
14-
May -09 |
| US20090118
503 |
FAAH INHIBITORS | Not Available | 20-
Jun- 07 |
20-
Jun -08 |
7-
May -09 |
| US20090118
288 |
N-Benzyl-Morpholine Derivatives as Modulators of the Chemokine Receptor | ASTRAZENECA AB | 21-
Jul-05 |
19-
Jul- 06 |
7-
May -09 |
| US20090118
263 |
Novel Adenine Compound | ASTRAZENECA AKTIEBOLAG | 22-
Sep- 05 |
20-
Sep -06 |
7-
May -09 |
| US20090117
537 |
METHOD FOR DETECTING SARS CORONAVIRUS | EIKEN KAGAKU KABUSHIKI KAISHA | 27-
Jun- 03 |
10-
Jun -08 |
7-
May -09 |
| US20090117
367 |
ARTICLE AND ASSOCIATED METHOD | GENERAL ELECTRIC COMPANY | 28-
Sep- 07 |
28-
Sep -07 |
7-
May -09 |
| US20090117
123 |
IMMUNOPEPTIDES OF HPV E6 AND E7 PROTEINS | National Health Research Institutes | 2-
Nov- 07 |
23-
Sep -08 |
7-
May -09 |
| US20090117
115 |
Binary epitope antibodies and B cell superantigen immune stimulants | NISHIYAMA YASUHIRO | 9-
Nov- 06 |
9-
Nov -07 |
7-
May -09 |
| US20090117
113 |
Immunogenic And Therapeutic Compositions For Streptococcus Pyogenes | Chiron Corporation | 8-
Oct- 04 |
11-
Oct -05 |
7-
May -09 |
| US20090111
828 |
L-ALANINE DERIVATIVES | ASTRAZENECA AB | 23-
Nov- 05 |
22-
Nov -06 |
30-
Apr- 09 |
| US20090111
091 |
SPECIMEN PRETREATMENT LIQUID, KIT FOR MEASURING VIRUS, AND METHOD FOR DETECTING VIRUS | SYSMEX CORPORATION | 31-
Oct- 07 |
31-
Oct -08 |
30-
Apr- 09 |
| US20090105
295 |
HYDROXYLAMINE SUBSTITUTED IMIDAZOQUINOLINES | Coley Pharmaceutical Group, Inc. | 14-
Nov- 03 |
12-
Nov -04 |
23-
Apr- 09 |
| US20090105
212 |
NOVEL ADENINE COMPOUND | AstraZeneca Aktiebolag a corporation of Sweden | 22-
Sep- 05 |
22-
Sep -06 |
23-
Apr- 09 |
| US20090105
203 |
COMPOUNDS FOR TREATING VIRAL INFECTIONS | Myriad Genetics, Incorporated | 16-
Oct- 06 |
16-
Oct -07 |
23-
Apr- 09 |
| US20090105
151 |
AMPHIPATHIC ALPHA-HELICAL PEPTIDE COMPOSITIONS AS ANTIVIRAL AGENTS | CHEONG KWANG HO | 19-
Jul-07 |
14-
Jul- 08 |
23-
Apr- 09 |
| US20090105
092 |
VIRAL DATABASE METHODS | THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK | 28-
Nov- 06 |
27-
Nov -07 |
23-
Apr- 09 |
| US20090104
226 |
Alphavirus Vectors for Respiratory Pathogen Vaccines | NOVARTIS VACCINES AND DIAGNOSTICS INC. | 21-
May- 04 |
20-
May -05 |
23-
Apr- 09 |
| US20090104
209 |
METHODS OF REDUCING A VIRAL INFECTION AND KITS THEREFORE | LABONTE PATRICK | 21-
Sep- 07 |
19-
Sep -08 |
23-
Apr- 09 |
| US20090104
147 |
Sialoadhesin-Related Compositions and Methods | DELPUTTE PETER | 11-
May- 06 |
11-
May -07 |
23-
Apr- 09 |
| US20090099
216 |
NOVEL ADENINE COMPOUND | AstraZeneca Aktiebolag A corporation of Sweden | 22-
Sep- 05 |
22-
Sep -06 |
16-
Apr- 09 |
| US20090099
161 |
Substituted Imidazoquinolines and Imidazonaphthyridines | Coley Pharmaceutial Group, Inc. | 11-
Feb- 05 |
10-
Feb -06 |
16-
Apr- 09 |
| US20090099
156 |
Heterocyclic Compounds as Ccr2b antagonists | BOWER JUSTIN FAIRFIELD | 24-
Dec- 04 |
19-
Dec -05 |
16-
Apr- 09 |
| US20090099
110 |
Antiviral oligonucleotides | JUTEAU JEAN-MARC | 13-
Sep- 02 |
28-
Feb -08 |
16-
Apr- 09 |
| US20090099
043 |
Construction of pool of interfering nucleic acids covering entire RNA target sequence and related compositions | ZHU YORK YUAN YUAN | 23-
Jul-07 |
21-
Nov -08 |
16-
Apr- 09 |
| US20090098
530 |
Cell Line For Producing Coronaviruses | CRUCELL HOLLAND B.V. | 22-
Jul-05 |
21-
Jul- 06 |
16-
Apr- 09 |
| US20090098
207 |
Technology for the Preparation of Microparticles | NexBio, Inc. | 24-
Jul-07 |
24-
Jul- 08 |
16-
Apr- 09 |
| US20090092
962 |
METHOD FOR DETECTING SARS CORONAVIRUS | EIKEN KAGAKU KABUSHIKI KAISHA | 27-
Jun- 03 |
10-
Jun -08 |
9-
Apr- 09 |
| US20090092
633 |
Polyamino acid for use as adjuvant | AKASHI MITSURU | 20-
Apr- 05 |
19-
Apr -06 |
9-
Apr- 09 |
| US20090092
581 |
Interferons of rhesus and cynomolgus origin and uses thereof | CLARK WILLIAM A | 11-
Apr- 07 |
11-
Apr -08 |
9-
Apr- 09 |
| US20090083
865 |
Transgenic Mouse Lines Expressing Human Ace2 and Uses Thereof | CHAN TEH-SHENG | 11-
Jan- 06 |
11-
Jan -07 |
26-
Mar -09 |
| US20090082
547 |
COMPOSITIONS FOR ENHANCING TRANSPORT OF MOLECULES INTO CELLS | IVERSEN PATRICK L | 29-
Apr- 03 |
5-
Nov -08 |
26-
Mar -09 |
| US20090082
332 |
PURINE DERIVATIVES FOR THE TREATMENT OF VIRAL OR ALLERGIC
DISEASES AND CANCERS |
ABBOT PHILIP | 22-
Sep- 05 |
20-
Sep -06 |
26-
Mar -09 |
| US20090082
287 |
METHODS OF ENHANCING MUCOSAL HYDRATION AND MUCOSAL CLEARANCE BY TREATMENT WITH SODIUM CHANNEL BLOCKERS AND OSMOLYTES | PARION SCIENCES, INC. | 7-
Sep- 06 |
7-
Sep -07 |
26-
Mar -09 |
| US20090081
675 |
METHODS, COMPOUNDS AND SYSTEMS FOR DETECTING A MICROORGANISM IN A SAMPLE | COLSTON JR BILL W | 24-
Aug- 07 |
21-
Aug -08 |
26-
Mar -09 |
| US20090081
252 |
DECREASING POTENTIAL IATROGENIC RISKS ASSOCIATED WITH INFLUENZA VACCINES | CHIRON BEHRING GMBH & CO. | 9-
Sep- 04 |
9-
Sep -05 |
26-
Mar -09 |
| US20090081
157 |
Immunostimulatory Combinations for Vaccine Adjuvants | Not Available | 9-
Jan- 06 |
9-
Jan -07 |
26-
Mar -09 |
| US20090078
263 |
Hazardous substance removing method, hazardous substance removing material used therein such as air filter, mask, wipe sheet, and the like, and storage method thereof | ARAJ JUN-ICHIRO | 28-
Mar- 03 |
21-
Nov -08 |
26-
Mar -09 |
| US20090076
076 |
INHIBITORS OF CYSTEINE PROTEASES AND METHODS OF USE THEREOF | Baylor University | 13-
Jun- 07 |
13-
Jun -08 |
19-
Mar -09 |
| US20090075
980 |
Pyrazolopyridines and Analogs Thereof | Coley Pharmaceutical Group, Inc. | 3-
Oct- 03 |
31-
Mar -06 |
19-
Mar -09 |
| US20090074
810 |
Modified Adenovirus Hexon Protein and Uses Thereof | The Trustees of the University of Pennsylvania | 28-
Apr- 06 |
27-
Apr -07 |
19-
Mar -09 |
| US20090069
314 |
Hydroxyalkyl Substituted Imidazoquinoline Compounds and Methods | Coley Pharmaceutical Group, Inc. | 23-
Feb- 05 |
22-
Feb -06 |
12-
Mar -09 |
| US20090068
759 |
REUSABLE DETECTION SURFACES AND METHODS OF USING SAME | BioScale, Inc. | 6-
Sep- 07 |
5-
Sep -08 |
12-
Mar -09 |
| US20090068
665 |
METHODS AND KITS FOR IDENTIFYING TARGET NUCLEOTIDES IN MIXED POPULATIONS | APPLIED BIOSYSTEMS INC. | 30-
Apr- 04 |
31-
Jul- 08 |
12-
Mar -09 |
| US20090068
636 |
VIRAL PROTEIN | CHANG MING-FU | 2-
Mar- 04 |
19-
Sep -07 |
12-
Mar -09 |
| US20090068
142 |
COMPOSITIONS AND METHODS FOR TREATING CORONAVIRUS INFECTION AND SARS | Three Rivers Pharmaceuticals LLC | 1-
Apr- 03 |
29-
Jul- 08 |
12-
Mar -09 |
| US20090068
095 |
CARBONIC ANHYDRASE IX (G250) ANITBODIES AND METHODS OF USE THEREOF | LO AGNES | 2-
Dec- 05 |
4-
Dec -06 |
12-
Mar -09 |
| US20090062
328 |
Oxime and Hydroxylamine Substituted Imidazo[4,5-c] Ring Compounds and Methods | COLEY PHARMACEUTICAL GROUP, INC. | 11-
Feb- 05 |
10-
Feb -06 |
5-
Mar -09 |
| US20090062
272 |
IMIDAZOQUINOLINYL SULFONAMIDES | BONK JASON D | 30-
Dec- 03 |
23-
Dec -04 |
5-
Mar -09 |
| US20090062
259 |
Bezothiazol Derivatives as Beta2 Adrenoreceptor Agonists | ASTRAZENECA AB | 14-
Mar- 06 |
12-
Mar -07 |
5-
Mar -09 |
| US20090062
229 |
METHOD AND COMPOSITION FOR REDUCING THE EXPRESSION OF ROCK-II | Myriad Genetics, Incorporated | 20-
Aug- 03 |
6-
May -08 |
5-
Mar -09 |
| US20090061
027 |
Composition For The Prevention and Treatment Of Common Cold Diseases | PANDALIS GEORGIOS | 24-
Mar- 06 |
2-
Mar -07 |
5-
Mar -09 |
| US20090061
017 |
SHELF STABLE, REDUCED CORROSION, READY TO USE PEROXYCARBOXYLIC ACID ANTIMICROBIAL COMPOSITIONS | BESSE MICHAEL | 30-
Aug- 07 |
30-
Aug -07 |
5-
Mar -09 |
| US20090060
950 |
Method for Producing Viral Vaccines | BAXTER HEALTHCARE | 28-
Aug- 07 |
28-
Aug -08 |
5-
Mar -09 |
| US20090054
413 |
Novel 5,6-Dihydropyrazolo[3,4-E] [L,4]Diazepin-4 (IH) -One
Derivatives for the T reatment of Asthma and Chronic Obstructive Pulmonary Disease |
AstraZeneca AB | 3-
Oct- 05 |
2-
Oct -06 |
26-
Feb -09 |
| US20090054
342 |
Bioactive peptides and method of using same | AYALON-SOFFER MICHAL | 18-
Sep- 06 |
18-
Sep -07 |
26-
Feb -09 |
| US20090053
708 |
Method and/or Apparatus of Oligonucleotide Design and/or Nucleic Acid Detection | LEE CHARLIE | 12-
Aug- 05 |
8-
Aug -06 |
26-
Feb -09 |
| US20090053
299 |
Methods for generating immune response using cationic-liposome- mediated nucleic acid delivery | Georgetown University | 9-Jul-
07 |
9-
Jul- 08 |
26-
Feb -09 |
| US20090053
257 |
REPLIKIN PEPTIDES AND USES THEREOF | BOGOCH ELENORE S | 6-
Jun- 03 |
10-
Jul- 08 |
26-
Feb -09 |
| US20090053
248 |
Compositions and methods for transepithelial molecular transport | Thomas Jefferson University | 31-
May- 02 |
25-
Mar -08 |
26-
Feb -09 |
| US20090047
665 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF ADENOVIRUSES | IBIS BIOSCIENCES, INC. | 11-
Sep- 03 |
16-
Sep -08 |
19-
Feb -09 |
| US20090047
353 |
CHANGING TH1/TH2 BALANCE IN SPLIT INFLUENZA VACCINES WITH ADJUVANTS | NOVARTIS VACCINES AND DIAGNOSTICS SRL | 4-
Nov- 05 |
6-
Nov -06 |
19-
Feb -09 |
| US20090047
306 |
ADJUVANT COMPOSITIONS | M N L PHARMA LIMITED | 21-
Jan- 04 |
21-
Jan -05 |
19-
Feb -09 |
| US20090047
272 |
Compositions with Modified Nucleases Targeted to Viral Nucleic Acids and Methods of Use for Prevention and Treatment of Viral Diseases | APPELBAUM JACOB G | 14-
Apr- 04 |
14-
Apr -05 |
19-
Feb -09 |
| US20090042
942 |
Muscarinic Receptor Antagonists | ASTRAZENECA AB | 20-
Apr- 05 |
18-
Apr -06 |
12-
Feb -09 |
| US20090042
925 |
OXIME SUBSTITUTED IMIDAZOQUINOLINES | Coley pharmaceutical Group, Inc. | 14-
Nov- 03 |
12-
Nov -04 |
12-
Feb -09 |
| US20090042
858 |
LACTAM CONTAINING HCV INHIBITORS | BARSANTI PAUL | 16-
Jun- 05 |
16-
Jun -06 |
12-
Feb -09 |
| US20090042
827 |
ANTIVIRAL OLIGONUCLEOTIDES TARGETING HBV | JUTEAU JEAN-MARC | 13-
Sep- 02 |
10-
Jul- 08 |
12-
Feb -09 |
| US20090042
292 |
B7-DC Variants | The Johns Hopkins University | 13-
Jul-07 |
11-
Jul- 08 |
12-
Feb -09 |
| US20090042
274 |
Method of Purifying Virus Envelope | GENOMIDEA INC. | 27-
Jul-04 |
22-
Jul- 05 |
12-
Feb -09 |
| US20090042
252 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | Maxygen, Inc. | 18-
Nov- 02 |
30-
Oct -06 |
12-
Feb -09 |
| US20090041
818 |
ANTIVIRAL AGENT, AND FABRIC AND ANTIVIRAL MEMBER SUPPORTING ANTIVIRAL AGENT | ITO HIROSHI | 12-
Aug- 03 |
10-
Aug -04 |
12-
Feb -09 |
| US20090041
803 |
Dioscorea Extracts | Academia Sinica | 1-
Dec- 03 |
2-
Sep -08 |
12-
Feb -09 |
| US20090041
781 |
METHODS FOR DETECTING PARVOVIRUS INFECTIONS | The Research Foundation of State University of New York | 5-
May- 03 |
16-
Oct -08 |
12-
Feb -09 |
| US20090036
653 |
Methods for the directed expansion of epitopes for use as antibody ligands | Peptimmune, Inc. | 13-
Apr- 06 |
7-
May -08 |
5-
Feb -09 |
| US20090036
535 |
Biphenyloxyacetic Acid Derivatives for the Treatment of Respiratory Disease | ASTRAZENECA AB | 6-
Oct- 05 |
5-
Oct -06 |
5-
Feb -09 |
| US20090035
861 |
RNAi Medicine Having No Adverse Effects | GONDAI TAKUMA | 28-
Jan- 05 |
27-
Jan -06 |
5-
Feb -09 |
| US20090035
323 |
IMMUNE RESPONSE MODIFIER CONJUGATES | Not Available | 22-
Feb- 06 |
21-
Feb -07 |
5-
Feb -09 |
| US20090012
280 |
OLIGONUCLEOTIDE COMPOUND AND METHOD FOR TREATING NIDOVIRUS INFECTIONS | BESTWICK RICHARD K | 24-
Dec- 03 |
25-
Apr -08 |
8-
Jan- 09 |
| US20090012
151 |
Novel Compounds 951 | BONNERT ROGER VICTOR | 5-Jul-
07 |
3-
Jul- 08 |
8-
Jan- 09 |
| US20090012
125 |
Piperidine Derivatives, Their Process for Preparation, Their Use as Therapeutic Agents and Pharmaceutical Compositions Containing Them | CAGE PETER | 7-
Mar- 06 |
6-
Mar -07 |
8-
Jan- 09 |
| US20090011
465 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | MAXYGEN, INC. | 18-
Nov- 02 |
30-
Oct -06 |
8-
Jan- 09 |
| US20090011
403 |
MICROPOROUS MATERIALS, METHODS OF MAKING, USING, AND ARTICLES THEREOF | UNIVERSITY OF UTAH RESEARCH FOUNDATION | 26-
May- 04 |
17-
May -05 |
8-
Jan- 09 |
| US20090005
376 |
1-Alkoxy lH-Imidazo Ring Systems and Methods | 3M Innovative Properties Company | 2-
Sep- 04 |
1-
Sep -05 |
1-
Jan- 09 |
| US20090005
371 |
Substituted Fused [1,2]Imidazo[4,5-C] Ring Compounds and Methods | HEPPNER PHILIP D | 11-
Feb- 05 |
10-
Feb -06 |
1-
Jan- 09 |
| US20090004
643 |
METHODS FOR CONCURRENT IDENTIFICATION AND QUANTIFICATION OF AN UNKNOWN BIOAGENT | ISIS Pharmaceuticals, Inc. | 18-
Feb- 04 |
17-
Feb -05 |
1-
Jan- 09 |
| US20080319
170 |
Methods and Uses of Antibodies in the Purification of Interferon | ViraNative AB | 12-
Feb- 05 |
13-
Feb -06 |
25-
Dec -08 |
| US20080318
998 |
Alkyloxy Substituted Thiazoloquinolines and Thiazolonaphthyridines | COLEY PHARMACEUTICAL GROUP, INC. | 9-
Feb- 05 |
8-
Feb -06 |
25-
Dec -08 |
| US20080317
702 |
METHOD FOR TREATING MICROORGANISMS AND/OR INFECTIOUS AGENTS | EDGINGTON GARRY | 19-
Jun- 07 |
19-
Jun -08 |
25-
Dec -08 |
| US20080311
138 |
Adjuvant Activity of Gastrointestinal Peptides | Not Available | 13-
Dec- 04 |
13-
Dec -05 |
18-
Dec -08 |
| US20080311
125 |
Scytovirin Domain 1 Related Polypeptides | Office of Technology Transfer | 25-
May- 05 |
24-
May -06 |
18-
Dec -08 |
| US20080311
042 |
Loop-Variant Pdz Domains as Biotherapeutics, Diagnostics and Research Reagents | DELAGRAVE SIMON | 5-
Dec- 05 |
4-
Dec -06 |
18-
Dec -08 |
| US20080310
992 |
APPARATUS AND METHOD FOR USING OZONE AS A DISINFECTANT | VIROFORCE SYSTEMS INC. | 29-
Nov- 06 |
17-
Jun -08 |
18-
Dec -08 |
| US20080305
120 |
Immunogenic Compositions Comprising Hmgb 1 Polypeptides | MEDIMMUNE, INC. | 17-
Jun- 04 |
16-
Jun -05 |
11-
Dec -08 |
| US20080305
119 |
Modified Bacteriophage Vectors and Uses Thereof | UNIVERSITY OF ROCHESTER | 29-
Oct- 04 |
5-
Oct -05 |
11-
Dec -08 |
| US20080300
275 |
Novel Benzothiazolone Derivatives | ASTRAZENECA AB | 9-
Aug- 05 |
3-
Aug -06 |
4-
Dec -08 |
| US20080300
244 |
NOVEL COMPOUNDS | AstraZeneca AB | 14-
Dec- 06 |
13-
Dec -07 |
4-
Dec -08 |
| US20080300
191 |
PROTEASE INHIBITORS FOR CORONAVIRUSES AND SARS-COV AND THE USE THEREOF | CAI SUI XIONG | 6-
May- 03 |
6-
May -04 |
4-
Dec -08 |
| US20080299
545 |
CHROMATOGRAPHIC METHODS FOR ASSESSING ADENOVIRUS PURITY | CLARKE PETER | 6-
Mar- 07 |
6-
Mar -08 |
4-
Dec -08 |
| US20080299
182 |
METHODS AND FORMULATIONS FOR TOPICAL GENE THERAPY | ONISHI ERIC | 1-
Mar- 07 |
29-
Feb -08 |
4-
Dec -08 |
| US20080299
070 |
Antiviral Compounds | ENGEL ROBERT | 30-
May- 07 |
30-
May -08 |
4-
Dec -08 |
| US20080295
843 |
SELF SANITIZING FACE MASKS AND METHOD OF MANUFACTURE | HAAS MARCI B | 1-
Jun- 07 |
1-
Jun -07 |
4-
Dec -08 |
| US20080293
775 |
Substituted Diphenylethers, -Amines, -Sulfides and -Methanes for the Treatment of Respiratory Disease | ASTRAZENECA AB | 15-
Dec- 05 |
12-
Dec -06 |
27-
Nov -08 |
| US20080293
742 |
Novel N-(Fluoro-Pyrazinyl)-Phenylsulfonamides as Modulators of Chemokine Receptor Ccr4 | CHESHIRE DAVID | 12-
Dec- 05 |
11-
Dec -06 |
27-
Nov -08 |
| US20080292
657 |
PRIMATE T-LYMPHOTROPIC VIRUSES | Centers for Disease Control and Prevention | 21-
Feb- 05 |
24-
Feb -07 |
27-
Nov -08 |
| US20080292
641 |
Antigenic GM-CSF peptides and antibodies to GM-CSF | Morphotek, Inc. | 8-
Feb- 06 |
8-
Feb -07 |
27-
Nov -08 |
| US20080287
453 |
Piperazine Compounds Useful as Antagonists of C-C Chemokines (Ccr2b and Ccr5) for the Treatment of Inflammatory Diseases | BOWER JUSTIN FAIRFIELD | 21-
Dec- 05 |
18-
Dec -06 |
20-
Nov -08 |
| US20080286
756 |
COMPOSITIONS AND METHODS FOR DIAGNOSING AND TREATING SEVERE ACUTE RESPIRATORY SYNDROME (SARS) | THE CHINESE UNIVERSITY OF HONG KONG | 29-
Jul-03 |
3-
Apr -08 |
20-
Nov -08 |
| US20080280
951 |
Salt Il | ASTRAZENECA AB | 2-
Aug- 05 |
31-
Jul- 06 |
13-
Nov -08 |
| US20080279
920 |
Compositions For Treating Respiratory Viral Infections and Their Use | Intradigm Corporation | 5-
Nov- 04 |
4-
Nov -05 |
13-
Nov -08 |
| US20080279
891 |
Viral Adjuvants | JOHNSTON ROBERT E | 9-Jul-
04 |
8-
Jul- 05 |
13-
Nov -08 |
| US20080279
812 |
Disease Prevention and Vaccination Prior to Thymic Reactivation | Monash University | 5-
Dec- 03 |
19-
Apr -04 |
13-
Nov -08 |
| US20080279
722 |
TRANSPORTABLE DECONTAMINATION UNIT AND DECONTAMINATION PROCESS | BACIK MICHAEL A | 6-
Mar- 07 |
20-
Feb -08 |
13-
Nov -08 |
| US20080279
721 |
DECONTAMINATION UNIT AND PROCESS | CENTANNI MICHAEL A | 6-
Mar- 07 |
20-
Feb -08 |
13-
Nov -08 |
| US20080279
720 |
DECONTAMINATION UNIT WITH COLLAPSIBLE DECONTAMINATION ENCLOSURE AND DECONTAMINATION PROCESS | CENTANNI MICHAEL A | 6-
Mar- 07 |
20-
Feb -08 |
13-
Nov -08 |
| US20080275
084 |
Piperidines for the Treatment of Chemokine Mediated Diseases | ASTRAZENECA AB | 27-
May- 05 |
24-
May -06 |
6-
Nov -08 |
| US20080274
140 |
Vaccines and Methods for Using the Same | KUTZLER MICHELE | 19-
Nov- 04 |
18-
Nov -05 |
6-
Nov -08 |
| US20080269
240 |
Novel Adenine Compound | AstraZeneca Aktiebolag A Corporation of Sweden | 22-
Sep- 05 |
21-
Sep -06 |
30-
Oct- 08 |
| US20080269
192 |
Chiral Fused [1,2]Imidazo[4,5-C] Ring Compounds | Coley Pharmaceutical Group, Inc. | 30-
Dec- 04 |
29-
Dec -05 |
30-
Oct- 08 |
| US20080269
156 |
Inhibitors of RTP801 and their use in disease treament | FEINSTEIN ELENA | 26-
Feb- 07 |
26-
Feb -08 |
30-
Oct- 08 |
| US20080269
148 |
Modified Small Interfering Rna Molecules and Methods of Use | HAN JANG | 1-
Oct- 04 |
30-
Sep -05 |
30-
Oct- 08 |
| US20080269
115 |
Immunogenic Sars Domain | BEADENKOPF ROBERT J | 17-
Jun- 04 |
16-
Jun -05 |
30-
Oct- 08 |
| US20080267
997 |
Modified Viral Particles with Immunogenic Properties and Reduced Lipid Content Useful for Treating and Preventing Infectious Diseases | Lipid Sciences, Inc. | 29-
Jun- 00 |
9-
May -08 |
30-
Oct- 08 |
| US20080267
992 |
Sars Virus Vaccine with Adenovirus Carrier and Preparation Method Thereof, and Use of Sars Virus S Gene for Preparation of Vaccine | Cancer Center, Sun Yat-Sun University | 4-
Jun- 04 |
4-
Jun -04 |
30-
Oct- 08 |
| US20080267
819 |
TRANSPORTABLE DECONTAMINATION UNIT AND DECONTAMINATION PROCESS | BACIK MICHAEL A | 6-
Mar- 07 |
20-
Feb -08 |
30-
Oct- 08 |
| US20080261
258 |
Immune Cell Biosensors and Methods of Using Same | Amaox, Inc. | 9-Jul-
04 |
11-
Jul- 05 |
23-
Oct- 08 |
| US20080261
257 |
Fluorescent Proteins and Related Methods and Compounds | UNIVERSITY OF MASSACHUSETTS | 20-
Sep- 04 |
20-
Sep -05 |
23-
Oct- 08 |
| US20080260
775 |
New Live Virus Vaccines | JOHNSON PHILIP R | 15-
Feb- 05 |
15-
Feb -06 |
23-
Oct- 08 |
| US20080260
773 |
Saccharide Conjugate Vaccines | Not Available | 24-
Dec- 04 |
23-
Dec -05 |
23-
Oct- 08 |
| US20080260
769 |
Polypeptides for Oligomeric Assembly of Antigens | CAPECCHIBARBARA | 23-
Jul-04 |
22-
Jul- 05 |
23-
Oct- 08 |
| US20080260
764 |
REPLIKIN PEPTIDES AND USES THEREOF | BOGOCH ELENORE S | 30-
May- 06 |
30-
May -07 |
23-
Oct- 08 |
| US20080255
150 |
Novel Compounds | ASTRAZENECA AB | 5-
Nov- 05 |
1-
Nov -06 |
16-
Oct- 08 |
| US20080255
076 |
Steroid-Derived Pharmaceutical Compositions | JadoLabs GmbH | 29-
Jun- 04 |
29-
Jun -05 |
16-
Oct- 08 |
| US20080254
440 |
Anti-Sars Virus Antibody, Hybridoma Producing the Antibody and Immunoassay Reagent Using the Antibody | FUJII NOBUYUKI | 31-
Oct- 03 |
29-
Oct -04 |
16-
Oct- 08 |
| US20080249
145 |
Salts 668 | WHITTOCK ROBERT | 8-
Feb- 07 |
7-
Feb -08 |
9-
Oct- 08 |
| US20080249
110 |
Novel Substituted 3-Sulfur Indoles | BONNERT ROGER | 27-
May- 03 |
25-
May -04 |
9-
Oct- 08 |
| US20080249
097 |
PRODRUGS OF HETEROARYL COMPOUNDS | Koronis Pharmaceuticals, Incorporation | 20-
Jun- 03 |
27-
Dec -06 |
9-
Oct- 08 |
| US20080249
039 |
Modified Short Interfering Rna (Modified Sirna) | SANTARIS PHARMA A/S | 30-
Jan- 04 |
28-
Jan -05 |
9-
Oct- 08 |
| US20080248
551 |
METHODS AND COMPOSITIONS FOR LIVE ATTENUATED VIRUSES | OSORIO JORGE E | 6-
Apr- 07 |
4-
Apr -08 |
9-
Oct- 08 |
| US20080248
043 |
Antibodies to SARS coronavirus | Amgen Inc. | 19-
May- 06 |
21-
May -07 |
9-
Oct- 08 |
| US20080242
851 |
MODIFIED POLYNUCLEOTIDES FOR REDUCING OFF-TARGET EFFECTS IN RNA INTERFERENCE | DHARMACON, INC. | 2-
Apr- 03 |
19-
Sep -07 |
2-
Oct- 08 |
| US20080242
794 |
COLOR STABILIZED ANTIMICROBIAL POLYMER COMPOSITES | BLANTON THOMAS N | 30-
Mar- 07 |
30-
Mar -07 |
2-
Oct- 08 |
| US20080242
649 |
New Combination 665 | CADOGAN ELAINE BRIDGET | 8-
Feb- 07 |
7-
Feb -08 |
2-
Oct- 08 |
| US20080241
935 |
Methods for pathogen detection | Searete LLC, a limited liability corporation of the State of Delaware | 27-
Mar- 07 |
27-
Mar -07 |
2-
Oct- 08 |
| US20080241
910 |
Devices for pathogen detection | Searete LLC, a limited liability corporation of the State of Delaware | 27-
Mar- 07 |
27-
Mar -07 |
2-
Oct- 08 |
| US20080241
909 |
Microfluidic chips for pathogen detection | Searete LLC, a limited liability corporation of the State of Delaware | 27-
Mar- 07 |
27-
Mar -07 |
2-
Oct- 08 |
| US20080241
511 |
PRODUCTION OF SILVER SULFATE GRAINS USING INORGANIC ADDITIVES | BLANTON THOMAS N | 30-
Mar- 07 |
30-
Mar -07 |
2-
Oct- 08 |
| US20080241
189 |
Sequential Delivery Of Immunogenic Molecules Via Adenovirus And Adeno-Associated Virus-Mediated Administrations | The Trustees of the University of Pennsylvania | 28-
Apr- 04 |
27-
Apr -05 |
2-
Oct- 08 |
| US20080241
000 |
Systems for pathogen detection | Searete LLC, a limited liability corporation of the State of Delaware | 27-
Mar- 07 |
27-
Mar -07 |
2-
Oct- 08 |
| US20080234
345 |
METHOD FOR REDUCING OR ALLEVIATING INFLAMMATION IN THE DIGESTIVE TRACT | Gene Logic Inc. | 8-
Sep- 06 |
7-
Sep -07 |
25-
Sep -08 |
| US20080234
319 |
Novel Compounds 679 | EBDEN MARK | 22-
Mar- 07 |
21-
Mar -08 |
25-
Sep -08 |
| US20080233
650 |
Method for propagating adenoviral vectors encoding inhibitory gene products | GENVEC, INC. | 10-
Nov- 05 |
9-
May -08 |
25-
Sep -08 |
| US20080233
570 |
METHODS FOR IDENTIFICATION OF SEPSIS-CAUSING BACTERIA | HALL THOMAS A | 11-
Sep- 03 |
25-
May -07 |
25-
Sep -08 |
| US20080233
558 |
Inhibitors of viral entry screening method | Medical Research Council | 1-
Feb- 05 |
1-
Aug -07 |
25-
Sep -08 |
| US20080233
150 |
RESPIRATORY SYNCYTIAL VIRUS-VIRUS LIKE PARTICLE (VLPS) | MAHMOOD KUTUB | 16-
Nov- 06 |
16-
Nov -07 |
25-
Sep -08 |
| US20080233
128 |
Treatment of Viral Infections | KRAUSE WERNER | 2-
Nov- 06 |
1-
Nov -07 |
25-
Sep -08 |
| US20080227
817 |
New Salt I | ASTRAZENECA AB | 2-
Aug- 05 |
31-
Jul- 06 |
18-
Sep -08 |
| US20080227
797 |
Pyridopyrimidine Derivatives as Pde4 Inhibitors for the Treatment of Inflammatory and Immune Diseases | ASTRAZENECA AB | 4-Jul-
05 |
3-
Jul- 06 |
18-
Sep -08 |
| US20080227
219 |
Electrochemiluminescent assay | Not Available | 17-
Nov- 04 |
16-
Nov -05 |
18-
Sep -08 |
| US20080227
149 |
Interferon-Alpha Polypeptides and Conjugates | MAXYGEN, INC. | 18-
Nov- 02 |
25-
Oct -07 |
18-
Sep -08 |
| US20080226
681 |
Highly Active Glycoproteins-Process Conditions and an Efficient Method for their Production | Glycotope GmbH | 13-
Feb- 04 |
14-
Feb -05 |
18-
Sep -08 |
| US20080226
597 |
EVOLVED INTERFERON-ALPHA POLYPEPTIDES | MAXYGEN, INC. | 18-
May- 05 |
17-
May -06 |
18-
Sep -08 |
| US20080213
891 |
RNAi Agents Comprising Universal Nucleobases | Alnylam Pharmaceuticals, Inc. | 21-
Jul-04 |
6-
Aug -07 |
4-
Sep -08 |
| US20080213
308 |
Imidazoquinoline Compounds | CHU DANIEL | 14-
Sep- 04 |
14-
Sep -05 |
4-
Sep -08 |
| US20080213
284 |
RECEPTOR BINDING POLYPEPTIDES | National Health Research Institutes, a Taiwanese corporation | 9-
Jan- 04 |
10-
Jan -05 |
4-
Sep -08 |
| US20080213
125 |
Apparatus and Method for Using Ozone as a Disinfectant | Huawei Technologies Co. LTD. | 18-
Mar- 04 |
18-
Mar -05 |
4-
Sep -08 |
| US20080210
748 |
Systems and methods for receiving pathogen related information and responding | Searete LLC, a limited liability corporation of the State of Delaware, | 30-
Nov- 05 |
11-
Sep -07 |
4-
Sep -08 |
| US20080207
698 |
Novel Compounds 569 | CONNOLLY STEPHEN | 20-
Dec- 06 |
19-
Dec -07 |
28-
Aug -08 |
| US20080207
688 |
Novel Piperidine Derivatives | ASTRAZENECA AB | 21-
Jul-05 |
19-
Jul- 06 |
28-
Aug -08 |
| US20080207
674 |
Immune Response Modifier Formulations And Methods | Coley Pharmaceutical Group, Inc. | 30-
Dec- 04 |
28-
Dec -05 |
28-
Aug -08 |
| US20080207
650 |
Chemical Compounds 636 | BONNERT ROGER VICTOR | 11-
Jan- 07 |
10-
Jan -08 |
28-
Aug -08 |
| US20080207
597 |
HOMOPIPERAZINE COMPOUNDS THAT INHIBIT RIBOSOMAL FRAMESHIFTING BY BINDING TO RNA PSEUDOKNOT STRUCTURE OF SARS CORONAVIRUS | SUNGKYUNKWAN UNIVERSITY FOUNDATION FOR CORPORATE COLLABORATION | 22-
Dec- 06 |
20-
Dec -07 |
28-
Aug -08 |
| US20080207
596 |
Novel compounds 329 | MARTIN BARRIE | 19-
Jun- 06 |
19-
Jun -07 |
28-
Aug -08 |
| US20080207
587 |
Pyridazine Derivatives for Inhibiting Human Stearoyl-Coa- Desaturase | XENON PHARMACEUTICALS INC. | 20-
Sep- 04 |
20-
Sep -05 |
28-
Aug -08 |
| US20080207
573 |
COMPOUNDS FOR TREATING VIRAL INFECTIONS | Myriad Genetics, Incorporated | 16-
Oct- 06 |
7-
May -08 |
28-
Aug -08 |
| US20080206
283 |
Vaccine Against Sars | DONG JOHN Y | 17-
Jun- 03 |
17-
Jun -04 |
28-
Aug -08 |
| US20080206
235 |
COMPOSITIONS AND METHODS FOR STIMULATING AN IMMUNE RESPONSE | Johns Hopkins University | 27-
Dec- 06 |
27-
Dec -07 |
28-
Aug -08 |
| US20080200
505 |
Piperidines for the Treatment of Chemokine Mediated Diseases | ASTRAZENECA AB | 27-
May- 05 |
24-
May -06 |
21-
Aug -08 |
| US20080199
915 |
Methods and Kits For Mass Production Of Dsrna | RNA-Line Oy | 2-
May- 03 |
2-
May -03 |
21-
Aug -08 |
| US20080199
495 |
Stimulation of thymus for vaccination development | Monash University | 15-
Apr- 99 |
24-
May -07 |
21-
Aug -08 |
| US20080199
491 |
Sustained Release Vaccine Composition | BRANDON MALCOLM | 16-
Jun- 04 |
16-
Jun -05 |
21-
Aug -08 |
| US20080199
481 |
COMPOUNDS | ASTRAZENECA AB | 21-
Feb- 07 |
19-
Feb -08 |
21-
Aug -08 |
| US20080194
922 |
POTENTIATION FOR MEDICAL THERAPIES | HOLDEN JAMES F | 7-
Sep- 05 |
28-
Sep -07 |
14-
Aug -08 |
| US20080194
689 |
Disinfectant and Germicidal Agent | REICHWAGEN SVEN | 25-
May- 05 |
26-
May -06 |
14-
Aug -08 |
| US20080194
632 |
Novel Piperidine Derivatives as Chemokine Receptor Modulators Useful for the Treatment of Respiratory Diseases | ASTRAZENECA AB | 1-
Aug- 05 |
31-
Jul- 06 |
14-
Aug -08 |
| US20080194
481 |
Albumin Fusion Proteins | Human Genome Sciences, Inc. | 21-
Dec- 01 |
31-
Oct -07 |
14-
Aug -08 |
| US20080194
422 |
PCR PRIMER SET DETECTING SEVERE ACUTE RESPIRATORY SYNDROME (SARS)-CORONAVIRUS, METHOD AND KIT FOR DETECTING SARS-CORONAVIRUS USING THE SAME | SAMSUNG ELECTRONICS CO., LTD. | 12-
Dec- 03 |
21-
Apr -08 |
14-
Aug -08 |
| US20080193
919 |
Systems and methods for receiving pathogen related information and responding | Searete LLC, a limited liability corporation of the State of Delaware | 30-
Nov- 05 |
22-
Jan -08 |
14-
Aug -08 |
| US20080193
474 |
Immunostimulatory Compositions | GRAM CHRISTOPHER D | 25-
Apr- 05 |
25-
Apr -06 |
14-
Aug -08 |
| US20080188
513 |
1-(2-Methylpropyl)-1H-Imidazo[4,5-C](1,5]Naphthyridin-4-Amine
Ethanesulfonate and 1-(2-Methylpropyl)-1H-Imidazo[4,5- C](1,5]Naphthyridin-4-Amine Methanesulfonate |
taked Pharmaceutical Company Limited | 30-
Dec- 04 |
28-
Dec -05 |
7-
Aug -08 |
| US20080188
488 |
Heterocyclic Derivatives and Their Use as Stearoyl-Coa Desaturase Inhibitors | XENON PHARMACEUTICALS INC. | 20-
Sep- 04 |
20-
Sep -05 |
7-
Aug -08 |
| US20080187
609 |
Methods and apparatus to prevent, treat, and cure the symptoms of nausea caused by chemotherapy treatments of human cancers | VAIL MARILYN L | 3-
Apr- 00 |
18-
Mar -08 |
7-
Aug -08 |
| US20080187
528 |
ANTI-TSG101 ANTIBODIES AND THEIR USES FOR TREATMENT OF VIRAL INFECTIONS | Functional Genetics, Inc. | 15-
Nov- 06 |
15-
Nov -07 |
7-
Aug -08 |
| US20080183
396 |
Systems and methods for transmitting pathogen related information and responding | Searete LLC, a limited libility corporation of the State of Delaware | 30-
Nov- 05 |
11-
Sep -07 |
31-
Jul- 08 |
| US20080182
900 |
Method of treatment of virus infections using shikonin compounds | WANG FEIXIN | 21-
Feb- 03 |
26-
Sep -07 |
31-
Jul- 08 |
| US20080182
874 |
Novel Compounds | ASTRAZENECA AB | 30-
Nov- 04 |
28-
Nov -05 |
31-
Jul- 08 |
| US20080181
969 |
POLYMER COMPOSITE | BARNES CRAIG L | 31-
Jan- 07 |
31-
Jan -07 |
31-
Jul- 08 |
| US20080176
902 |
Salt III | ASTRAZENECA AB | 2-
Aug- 05 |
31-
Jul- 06 |
24-
Jul- 08 |
| US20080176
841 |
EPOXIDE INHIBITORS OF CYSTEINE PROTEASES | THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIVERSITY | 13-
Jun- 06 |
13-
Jun -07 |
24-
Jul- 08 |
| US20080176
217 |
METHOD OF PREDICTING INFLUENZA OUTBREAKS BY CORRELATING AN INCREASE IN REPLIKIN COUNT IN SHRIMP WHITE SPOT SYNDROME VIRUS AND/OR TAURA SYNDROME VIRUS | BOGOCH ELENORE S | 24-
Oct- 06 |
24-
Oct -07 |
24-
Jul- 08 |
| US20080175
861 |
MODIFIED POLYMERASES AND ATTENUATED VIRUSES AND METHODS OF USE THEREOF | THE PENN STATE RESEARCH FOUNDATION | 22-
Dec- 06 |
24-
Dec -07 |
24-
Jul- 08 |
| US20080175
832 |
Materials and Methods for Prevention and Treatment of RNA Viral Diseases | BEHERA ARUNA K | 30-
Apr- 02 |
17-
Jan -08 |
24-
Jul- 08 |
| US20080172
247 |
Method to Decrease the Risk of a Vaccine-Induced Chronic Immune Mediated Disorder in Humans With a Family History of the Disorder | CLASSEN IMMUNOTHERAPIES | 6-
Apr- 04 |
6-
Apr -05 |
17-
Jul- 08 |
| US20080171
363 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | MAXYGEN, INC. | 19-
May- 04 |
14-
Aug -07 |
17-
Jul- 08 |
| US20080171
066 |
Listeriolysin-Containing Bacillus Spores as Antigen Delivery Agents | CUTTING SIMON | 19-
Feb- 05 |
20-
Feb -06 |
17-
Jul- 08 |
| US20080171
057 |
Reagents, Devices, and Methods For Proteomic Analysis With Applications Including Diagnostics, Vaccines, Quality Control and Research | NETWORK IMMUNOLOGY INC. | 21-
Apr- 04 |
19-
Apr -05 |
17-
Jul- 08 |
| US20080170
996 |
Compositions and Methods for Stimulation of Lung Innate Immunity | The Board of Regents of the University of Texas System | 28-
Jul-06 |
30-
Jul- 07 |
17-
Jul- 08 |
| US20080167
332 |
Novel Compounds 243 | ELKINS BARRY | 19-
Jul-06 |
18-
Jul- 07 |
10-
Jul- 08 |
| US20080167
321 |
Pyridine Derivatives For Inhibiting Human Stearoyl-Coa-Desaturase | XENON PHARMACEUTICALS INC. | 20-
Sep- 04 |
20-
Sep -05 |
10-
Jul- 08 |
| US20080167
198 |
Filter based detection system | COONEY CHRISTOPHER GERARD | 4-
Jan- 07 |
4-
Jan -07 |
10-
Jul- 08 |
| US20080166
793 |
Sorting, amplification, detection, and identification of nucleic acid subsequences in a complex mixture | The Regents of the University of California | 4-
Jan- 07 |
4-
Jan -07 |
10-
Jul- 08 |
| US20080166
701 |
Immunoassay Method and Immunoassay Kit to Be Used Therein | ARKRAY, Inc. | 25-
Jul-05 |
24-
Jul- 06 |
10-
Jul- 08 |
| US20080166
370 |
Immunogenic Compositions Comprising Multiple Gonococcal Antigens | CHIRON SRL | 26-
Jun- 03 |
25-
Jun -04 |
10-
Jul- 08 |
| US20080160
010 |
Peptide That Elicits Neutralizing Antibodies Targeting the Hiv CoReceptor | The Government of the United States of America as | 9-
Apr- 04 |
11-
Apr -05 |
3-
Jul- 08 |
| US20080159
962 |
Use of Inhibitors of the Renin-Angiotensin System for the Treatment of Lung Injuries | IMBA-INSTITUTE FUR MOLEKULARE BIOTECHNOLOGIE GMBH | 19-
May- 05 |
19-
May -06 |
3-
Jul- 08 |
| US20080156
743 |
HAZARDOUS SUBSTANCE REMOVING MATERIAL, METHOD FOR REMOVING HAZARDOUS SUBSTANCES, AND NONWOVEN FABRIC | FUJIFILM Corporation | 27-
Dec- 06 |
27-
Dec -07 |
3-
Jul- 08 |
| US20080154
210 |
Mixture for Transdermal Delivery of Low and High Molecular Weight Compounds | ORYXE | 28-
May- 04 |
25-
May -05 |
26-
Jun- 08 |
| US20080153
850 |
Adamantyl Derivates as P2x7 Receptor Antagonists | AstraZeneca AB | 30-
Aug- 04 |
29-
Aug -05 |
26-
Jun- 08 |
| US20080153
837 |
Novel Fluorene Derivatives, Composition Containing Said Derivatives and the Use Thereof | AVENTIS PHARMA S.A. | 19-
May- 05 |
14-
Nov -07 |
26-
Jun- 08 |
| US20080152
728 |
Noble gas-chlorine mixture effective against micro organisms | GLOBUS ALFRED R | 7-Jul-
03 |
25-
Apr -07 |
26-
Jun- 08 |
| US20080152
544 |
Building decontamination with vaporous hydrogen peroxide | STERIS INC. | 29-
Jan- 04 |
3-
Mar -08 |
26-
Jun- 08 |
| US20080149
100 |
Antiviral Heat Treatment | DE HAAN PETRUS THEODORUS | 20-
Dec- 06 |
20-
Dec -06 |
26-
Jun- 08 |
| US20080146
612 |
Novel Biaromatic Compounds, Inhibitors of the P2X7-Receptor | ASTRAZENECA AB | 27-
Jan- 05 |
25-
Jan -06 |
19-
Jun- 08 |
| US20080146
455 |
METHODS FOR IDENTIFICATION OF SEPSIS-CAUSING BACTERIA | HALL THOMAS A | 11-
Sep- 03 |
25-
May -07 |
19-
Jun- 08 |
| US20080145
847 |
METHODS FOR IDENTIFICATION OF SEPSIS-CAUSING BACTERIA | HALL THOMAS A | 11-
Sep- 03 |
25-
May -07 |
19-
Jun- 08 |
| US20080138
808 |
METHODS FOR IDENTIFICATION OF SEPSIS-CAUSING BACTERIA | HALL THOMAS A | 11-
Sep- 03 |
25-
May -07 |
12-
Jun- 08 |
| US20080132
502 |
5-Heteroaryl Thiazoles And Their Use As PI3K Inhibitors | ARNOULD JEAN-CLAUDE | 9-
Nov- 04 |
7-
Nov -05 |
5-
Jun- 08 |
| US20080132
480 |
Biphenyloxyacetic Acid Derivatives for the Treatment of Respiratory Disease | AstraZeneca AB | 24-
Aug- 04 |
22-
Aug -05 |
5-
Jun- 08 |
| US20080131
465 |
Group a Streptococcus Crge Protein | MANETTI ANDREA | 27-
Sep- 04 |
27-
Sep -05 |
5-
Jun- 08 |
| US20080131
446 |
Vaccine Composition | SANOFI PASTEUR SA | 17-
Nov- 03 |
9-
Jan -08 |
5-
Jun- 08 |
| US20080125
434 |
Heterocyclic Derivatives and Their Use as Strearoyl-Coa Desaturase Inhibitors | XENON PHARMACEUTICALS INC. | 20-
Sep- 04 |
20-
Sep -05 |
29-
May -08 |
| US20080124
323 |
Nutritional Composition Comprising Indigestible Oligosaccharides | N.V. Nutricia | 24-
Aug- 04 |
24-
Aug -05 |
29-
May -08 |
| US20080124
311 |
Novel pharmaceutical compositions for the treatment of virus infection and cancer | Illumigen Biosciences, Inc. | 23-
Nov- 05 |
17-
Nov -06 |
29-
May -08 |
| US20080124
303 |
Methods and compositions for treatment of viral infections | Cavit Sciences, Inc | 12-
Dec- 05 |
12-
Jul- 07 |
29-
May -08 |
| US20080124
302 |
Uses of Recombinant Super-Compound Interferons | WEI GUANGWEN | 9-
Mar- 05 |
9-
Mar -06 |
29-
May -08 |
| US20080118
530 |
Modulation of Replicative Fitness By Deoptimization of Synonymous Codons | BURNS CARA C | 8-
Oct- 04 |
7-
Oct -05 |
22-
May -08 |
| US20080118
517 |
Human monoclonal antibodies against interleukin 8 (IL-8) | GENMAB A/S | 16-
Dec- 02 |
27-
Jun -07 |
22-
May -08 |
| US20080118
495 |
Mammalian Genes Involved in Infection | RUBIN DONALD H | 27-
Oct- 04 |
27-
Oct -05 |
22-
May -08 |
| US20080118
473 |
Methods of treating a respiratory condition comprising probiotic treatment | Alimentary Health Ltd. | 1-
Nov- 06 |
31-
Oct -07 |
22-
May -08 |
| US20080114
019 |
Hydroxylamine Substituted Imidazoquinolines | Coley Pharmaceutical Group, Inc. | 12-
Aug- 03 |
12-
Aug -04 |
15-
May -08 |
| US20080114
002 |
Substituted Acids for the Treatment of Respiratory Diseases | ASTRAZENECA AB | 8-Jul-
04 |
6-
Jul- 05 |
15-
May -08 |
| US20080113
409 |
Transient protein expression methods | HATEBOER GUUS | 15-
Apr- 99 |
1-
Jun -07 |
15-
May -08 |
| US20080113
337 |
Method of Examining/Judging Presence of Virus Infection such as HIV or Presence of Prion Infection by Near-Infrared Spectroscopy and Device Used in Same | OSAKA UNIVERSITY | 12-
Nov- 04 |
10-
Nov -05 |
15-
May -08 |
| US20080108
629 |
Heterocyclic Derivatives for the Treatment of Diseases Mediated by Stearoyl-Coa Desaturase Enzymes | XENON PHARMACEUTICALS INC. | 20-
Sep- 04 |
20-
Sep -05 |
8-
May -08 |
| US20080107
650 |
METHOD FOR TREATING INFLAMMATORY DISEASES OF THE DIGESTIVE TRACT | Gene Logic Inc. | 8-
Sep- 06 |
7-
Sep -07 |
8-
May -08 |
| US20080103
746 |
Systems and methods for pathogen detection and response | Searete LLC, a limited liability corporation | 30-
Nov- 05 |
11-
Sep -07 |
1-
May -08 |
| US20080103
149 |
NOVEL TETRACYCLIC INHIBITORS OF CYSTEINE PROTEASES, THE PHARMACEUTICAL COMPOSITIONS THEREOF AND THEIR THERAPEUTIC APPLICATIONS | COLLAND FREDERIC | 30-
Oct- 06 |
30-
Oct -06 |
1-
May -08 |
| US20080102
444 |
METHODS AND COMPOSITIONS FOR DETECTING RHINOVIRUSES | Focus Technologies, Inc. | 6-Jul-
04 |
15-
Oct -07 |
1-
May -08 |
| US20080096
959 |
Durable Biocides and Disinfectants | MITSUI NORIN CO., LTD. | 7-Jul-
04 |
5-
Jul- 05 |
24-
Apr- 08 |
| US20080096
928 |
Methods for treating Hepatitis C | AREFOLOV ALEXANDER | 14- Jul-04 | 14-
Jul- 05 |
24-
Apr- 08 |
| US20080096
895 |
Heterocyclic Derivatives and Their Use as Stearoyl-Coa Desaturase Inhibitors | XENON PHARMACEUTICALS INC. | 20-
Sep- 04 |
20-
Sep -05 |
24-
Apr- 08 |
| US20080096
186 |
PCR PRIMER SET FOR DETECTING SEVERE ACUTE RESPIRATORY SYNDROME (SARS)-CORONAVIRUS, METHOD AND KIT FOR DETECTING SARS-CORONAVIRUS USING THE SAME | HWANG JUNG-JOO | 12-
Dec- 03 |
24-
Nov -04 |
24-
Apr- 08 |
| US20080090
913 |
Sphingolipid-Derived Phamaceutical Compositions | BRAXMEIER TOBIAS | 29-
Jun- 04 |
29-
Jun -05 |
17-
Apr- 08 |
| US20080090
841 |
METHODS OF ENHANCING MUCOSAL HYDRATION AND MUCOSAL CLEARANCE BY TREATMENT WITH SODIUM CHANNEL BLOCKERS AND OSMOLYTES | PARION SCIENCES, Inc. | 7-
Sep- 06 |
7-
Sep -07 |
17-
Apr- 08 |
| US20080090
791 |
Cystic fibrosis treatment methods | Hollis-Eden Pharmaceuticals, Inc. | 13-
Oct- 06 |
13-
Oct -06 |
17-
Apr- 08 |
| US20080090
229 |
Devices for generating detectable polymers | ENGELHARD ERIC K | 12-
Oct- 06 |
12-
Oct -06 |
17-
Apr- 08 |
| US20080090
224 |
Nucleic acid detection | HAI KANG LIFE CORPORATION LIMITED | 2-
May- 03 |
30-
Apr -04 |
17-
Apr- 08 |
| US20080085
895 |
Substituted Chiral Fused [1,2]Imidazo[4,5-C] Ring Compounds | DANIELSON MICHAEL M | 30-
Dec- 04 |
29-
Dec -05 |
10-
Apr- 08 |
| US20080085
873 |
Disease treatment methods | Hollis-Eden Pharmaceuticals, Inc. | 28-
Aug- 02 |
13-
Oct -06 |
10-
Apr- 08 |
| US20080081
047 |
Anti-Sars Monoclonal Antibodies | ANDONOV ANTON | 5-
Dec- 03 |
6-
Dec -04 |
3-
Apr- 08 |
| US20080076
710 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | MAXYGEN, INC. | 19-
May- 04 |
4-
May -07 |
27-
Mar -08 |
| US20080076
115 |
Compositions and Methods for Detecting Severe Acute Respiratory Syndrome Coronavirus | GILLIM-ROSS LAURA | 3-
Nov- 03 |
3-
Nov -04 |
27-
Mar -08 |
| US20080075
740 |
Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
31-
Oct -07 |
27-
Mar -08 |
| US20080075
737 |
Adeno-Associated Virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
6-
Sep -07 |
27-
Mar -08 |
| US20080075
693 |
METHODS FOR TREATING VIRAL INFECTION USING IL-28 AND IL- 29 CYSTEINE MUTANTS | ZymoGenetics, Inc. | 2-
Apr- 04 |
20-
Sep -07 |
27-
Mar -08 |
| US20080071
063 |
Protein Formulations | MedImmune, Inc. | 3-
Feb- 06 |
2-
Feb -07 |
20-
Mar -08 |
| US20080070
907 |
Substituted chiral fused [1,2] imidazo [4,5-C] ring compounds and methods | Coley Pharmaceutical Group, Inc. | 12-
Jul-06 |
12-
Jul- 07 |
20-
Mar -08 |
| US20080069
839 |
ISOLATION AND CHARACTERIZATION OF THE PRECURSOR VIRUS OF HUMAN SARS VIRUS: SARS-ASSOCIATED CORONA VIRUS-LIKE VIRUS | GUAN YI | 22-
May- 03 |
24-
May -04 |
20-
Mar -08 |
| US20080069
838 |
NOVEL HUMAN VIRUS CAUSING SEVERE ACUTE RESPIRATORY SYNDROME (SARS) AND USES THEREOF | CHAN KWOK HUNG | 24-
Mar- 04 |
24-
Mar -04 |
20-
Mar -08 |
| US20080069
836 |
METHOD OF USING ADENOVIRAL VECTORS WITH INCREASED IMMUNOGENICITY IN VIVO | and Human Services | 1-
Sep- 04 |
26-
Feb -07 |
20-
Mar -08 |
| US20080069
830 |
Dna Sequences, Peptides, Antibodies and Vaccines for Prevention and Treatment of Sars | HOFFMAN STEPHEN L | 24-
Jun- 03 |
24-
Jun -04 |
20-
Mar -08 |
| US20080069
804 |
Alphavirus Replicon Packaging Constructs | PERRI SILVIA | 25-
May- 04 |
20-
May -05 |
20-
Mar -08 |
| US20080064
105 |
Animal protein-free media for cultivation of cells | Baxter Healthcare Corporation | 29-
Oct- 04 |
30-
Oct -07 |
13-
Mar -08 |
| US20080064
080 |
Animal protein-free media for cultivation of cells | Baxter Healthcare Corporation | 29-
Oct- 04 |
30-
Oct -07 |
13-
Mar -08 |
| US20080063
664 |
High-yield transgenic mammalian expression system for generating virus-like particles | Academia Sinica | 5-
Sep- 06 |
5-
Sep -06 |
13-
Mar -08 |
| US20080058
309 |
Novel Compounds 171 | ASTRAZENECA AB | 27-
Jul-06 |
26-
Jul- 07 |
6-
Mar -08 |
| US20080057
513 |
METHOD FOR PRODUCING NUCLEIC ACID PROBES | Ventana Medical Systems, Inc. | 1-
Sep- 06 |
31-
Aug -07 |
6-
Mar -08 |
| US20080051
297 |
Method for determining antigen-specific T cell response in high throughput format | Centro di Biotecnologie Avanzate and istituto Giannina Gaslini | 12-
Jun- 06 |
11-
Jun -07 |
28-
Feb -08 |
| US20080050
718 |
Methods, Articles, and Compositions for Identifying Oligonucleotides | ATKINS JOHN F | 14-
Nov- 03 |
15-
Nov -04 |
28-
Feb -08 |
| US20080044
816 |
Assay for Sars Coronavirus by Amplification and Detection of the Replicase Sequence | BECTON, DICKINSON AND COMPANY | 12-
Sep- 03 |
13-
Sep -04 |
21-
Feb -08 |
| US20080044
814 |
Reagents and Methods for Detecting Severe Acute Respiratory Syndrome Coronavirus | HIBBERD MARTIN L | 21-
Apr- 03 |
21-
Apr -04 |
21-
Feb -08 |
| US20080044
438 |
Yeast Cell Particles As Oral Delivery Vehicles For Antigens | OSTROFF GARY R | 17-
Mar- 06 |
16-
Mar -07 |
21-
Feb -08 |
| US20080044
437 |
Encapsidation System for Production of Recombinant Virus-Like Particles | CHEN QUN | 2-
Sep- 04 |
1-
Sep -05 |
21-
Feb -08 |
| US20080044
426 |
Novel Atypical Pneumonia-Causing Virus | BESTEBROER THEODORUS MARINUS | 18-
Nov- 03 |
18-
Nov -04 |
21-
Feb -08 |
| US20080044
423 |
COMPOUNDS | COCHRANE DUNCAN | 23-
Jun- 06 |
22-
Jun -07 |
21-
Feb -08 |
| US20080044
384 |
Recombinant Human Cytomegalovirus And Vaccines Comprising Heterologous Antigens | Medlmmune Vaccines, Inc. | 25-
Jun- 04 |
24-
Jun -05 |
21-
Feb -08 |
| US20080044
378 |
Methods and Compositions for Protein Production Using Adenoviral Vectors | INTROGEN THERAPEUTICS, INC. | 15-
May- 06 |
15-
May -07 |
21-
Feb -08 |
| US20080033
172 |
Mutagenic Heterocycles | Koronis Pharmaceuticals, Incorporated | 19-
Dec- 03 |
10-
Dec -04 |
7-
Feb -08 |
| US20080031
979 |
USE OF EXTRACTS FOR THE TREATMENT OF VIRAL DISORDERS | BODDUPALLI SEKHAR | 4-
Aug- 06 |
4-
Aug -06 |
7-
Feb -08 |
| US20080031
877 |
Purification of bacterial antigens | COVACCI ANTONELLO | 17-
Feb- 06 |
16-
Feb -07 |
7-
Feb -08 |
| US20080031
868 |
HUMAN LYSOZYME MEDICINE, ITS MANUFACTURING METHOD AND APPLICATION THEREOF | AN MI | 9-
Jun- 05 |
9-
Jun -05 |
7-
Feb -08 |
| US20080031
853 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | ROCHE PALO ALTO LLC | 19-
May- 04 |
15-
Aug -07 |
7-
Feb -08 |
| US20080031
770 |
Apparatus and method for using ozone as a disinfectant | BOAST NIGEL | 2-
Aug- 06 |
29-
Nov -06 |
7-
Feb -08 |
| US20080027
092 |
1-Acetic Acid-Indole, -Indazole and -Benzimidazole Derivatives Useful for the Treatment of Respiratory Disorders | BONNERT ROGER VICTOR | 26-
Nov- 03 |
24-
Nov -04 |
31-
Jan- 08 |
| US20080027
006 |
Compositions And Methods For Modification And Prevention Of Sars Coronavirus Infectivity | The Regents of the University of Colorado | 12-
Feb- 04 |
14-
Feb -05 |
31-
Jan- 08 |
| US20080021
038 |
Novel Piperidine/8-Azabicyclo [3.2.1.] Octan Derivatives As Modulators Of Chemokine Receptor Ccr5 | FAULL ALAN | 24-
Jun- 04 |
20-
Jun -05 |
24-
Jan- 08 |
| US20080020
379 |
Diagnosis and prognosis of infectious diseases clinical phenotypes and other physiologic states using host gene expression biomarkers in blood | AGAN BRIAN K | 5-
Nov- 04 |
7-
Nov -05 |
24-
Jan- 08 |
| US20080015
247 |
QUERCETIN-CONTAINING COMPOSITIONS | LINES THOMAS C | 17-
Jul-06 |
16-
Jul- 07 |
17-
Jan- 08 |
| US20080015
230 |
HETEROCYCLIC DERIVATIVES AND THEIR USE AS THERAPEUTIC AGENTS | XENON PHARMACEUTICALS INC. | 20-
Sep- 04 |
20-
Sep -05 |
17-
Jan- 08 |
| US20080015
194 |
METHODS AND COMPOSITIONS OF TARGETED DRUG DEVELOPMENT | Joseph Errico | 23-
Jan- 06 |
23-
Jan -07 |
17-
Jan- 08 |
| US20080015
184 |
Urea Substituted Imidazopyridines, Imidazoquinolines, and Imidazonaphthyridines | 3M INNOVATIVE PROPERTIES COMPANY | 14-
Jun- 04 |
14-
Jun -05 |
17-
Jan- 08 |
| US20080014
204 |
Compositions Against Sars-Coronavirus and Uses Thereof | DE KRUIF CORNELIS A | 11-
Nov- 04 |
10-
Nov -05 |
17-
Jan- 08 |
| US20080014
183 |
Anticancer Agent Containing Dendritic Cell Having Rna Virus Transferred Thereinto | DNAVEC RESEARCH INC. | 24-
Jun- 04 |
28-
Apr -05 |
17-
Jan- 08 |
| US20080009
496 |
MUTAGENIC HETEROCYCLES | Koronis Pharmaceuticals, Inc. | 19-
Dec- 03 |
27-
Dec -06 |
10-
Jan- 08 |
| US20080009
040 |
ANIMAL PROTEIN-FREE MEDIA FOR CULTIVATION OF CELLS | Baxter Healthcare Corporation | 29-
Oct- 04 |
20-
Sep -07 |
10-
Jan- 08 |
| US20080004
236 |
High Dose, Short Interval Use of Sulfated Polysaccharides for Treatment of Infections | COMPER WAYNE D | 6-
Feb- 04 |
7-
Feb -05 |
3-
Jan- 08 |
| US20080004
228 |
New Expression Tools for Multiprotein Applications | BERGER IMRE | 9-
Mar- 04 |
25-
Nov -04 |
3-
Jan- 08 |
| US20080004
206 |
Albumin fusion proteins | BELL ADAM | 21-
Dec- 01 |
31-
Jul- 06 |
3-
Jan- 08 |
| US20080003
236 |
ADENOVIRUS FIBER SHAFT COMPOSITION AND METHODS OF USE | GenVec, Inc. | 18-
Oct- 04 |
13-
Apr -07 |
3-
Jan- 08 |
| US20070299
081 |
Heterocyclic Derivatives and Their Use as Mediators of Stearoyl-Coa Desaturase | XENON PHARMACEUTICALS INC. | 20-
Sep- 04 |
20-
Sep -05 |
27-
Dec -07 |
| US20070298
415 |
Method of Amplifying Nucleic Acid | Takara Bio Inc. | 10-
Dec- 03 |
6-
Dec -04 |
27-
Dec -07 |
| US20070292
453 |
RNA virus vaccines and methods | DITTMER DIRK P | 14-
Dec- 05 |
14-
Dec -06 |
20-
Dec -07 |
| US20070287
725 |
Isoxazole, Dihydroisoxazole, And Oxadiazole Substituted Imidazo Ring Compounds And Method | 3M INNOVATIVE PROPERTIES COMPANY | 18-
Jun- 04 |
17-
Jun -05 |
13-
Dec -07 |
| US20070287
724 |
Substituted Imidazoquinolines, Imidazopyridines, and Imidazonaphthyridines | 3M INNOVATIVE PROPERTIES COMPANY | 18-
Jun- 04 |
17-
Jun -05 |
13-
Dec -07 |
| US20070286
872 |
Live Attenuated Nidovirus Vaccines | Vanderbilt University | 22-
Apr- 03 |
22-
Apr -04 |
13-
Dec -07 |
| US20070286
822 |
Compounds for the Treatment of Periodontal Disease | ANACOR PHARMACEUTICALS INC. | 12-
Jun- 06 |
12-
Jun -07 |
13-
Dec -07 |
| US20070281
325 |
COMPOSITIONS AND METHODS FOR ANALYSIS OF TARGET ANALYTES | DANIELZADEH ROBERT | 17-
Sep- 03 |
20-
Jul- 06 |
6-
Dec -07 |
| US20070276
009 |
Compositions and Methods for Viral Inhibition | CHANG BRYAN | 15-
Oct- 03 |
15-
Oct -04 |
29-
Nov -07 |
| US20070275
938 |
Therapeutic Treatment Methods | AHLEM CLARENCE N | 28-
Aug- 02 |
12-
Feb -07 |
29-
Nov -07 |
| US20070275
937 |
Therapeutic Treatment Methods | AHLEM CLARENCE N | 28-
Aug- 02 |
12-
Feb -07 |
29-
Nov -07 |
| US20070275
883 |
2′-C-methyl-3′-O-L-valine ester ribofuranosyl cytidine for treatment of flaviviridae infections | GOSSELIN GILLES | 28-
Jun- 02 |
6-
Sep -06 |
29-
Nov -07 |
| US20070275
879 |
Use of Ulinastatin and Its Pharmaceutical Composition for Treating Severe Acute Respiratory Syndrome | GUANGDONG TECHPOOL BIOCHEM PHA | 26-
May- 03 |
25-
May -04 |
29-
Nov -07 |
| US20070275
465 |
Double-Stranded Ribonucleic Acid with Increased Effectiveness in an Organism | Alnylam Pharmaceuticals | 13-
Jun- 03 |
14-
Jun -04 |
29-
Nov -07 |
| US20070275
091 |
Compositions And Methods For Improved Mucus Function | KING MALCOLM | 30-
Mar- 04 |
30-
Mar -05 |
29-
Nov -07 |
| US20070275
002 |
Use Of Proteins And Peptides Encoded By The Genome Of A Novel Sars-Associated Coronavirus Strain | AZEBI SALIHA | 2-
Dec- 03 |
2-
Dec -04 |
29-
Nov -07 |
| US20070274
994 |
Virulence-Associated Adhesins | Novartis Vaccines and Diagnostics, Inc. | 26-
Jun- 03 |
25-
Jun -04 |
29-
Nov -07 |
| US20070274
983 |
Nutritional Composition Comprising Immunoglobulins and Oligosaccharides | NUTRICIA NV | 24-
Aug- 04 |
24-
Aug -05 |
29-
Nov -07 |
| US20070274
950 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | MAXYGEN INC | 18-
Nov- 02 |
19-
May -04 |
29-
Nov -07 |
| US20070274
922 |
Water Soluble Boronic Acid Fluorescent Reporter Compounds and Methods of Use Thereof | FANG HAO | 5-
Sep- 03 |
7-
Sep -04 |
29-
Nov -07 |
| US20070270
361 |
Sars Nucleic Acids, Proteins, Vaccines, and Uses Thereof | CHOU TE-HUI W | 4-
Aug- 03 |
4-
Aug -04 |
22-
Nov -07 |
| US20070270
360 |
Rna Interference Mediated Inhibition of Severe Acute Respiratory Syndrome (Sars) Gene Expression Using Short Interfering Nucleic Acid | SIRNA THERAPEUTICS, INC. | 15-
Apr- 03 |
13-
Apr -04 |
22-
Nov -07 |
| US20070266
957 |
Antipathogenic Domestic Livestock House, Disinfectants for Domestic Livestock House, Disinfectants for Living Organisms, Feedstuffs and Drinking Water for Animals | TAKAHASHI KAZUO | 28-
Sep- 04 |
25-
Aug -05 |
22-
Nov -07 |
| US20070265
236 |
Asthma Treatment Methods | AHLEM CLARENCE N | 28-
Aug- 02 |
16-
Oct -06 |
15-
Nov -07 |
| US20070265
226 |
Hydrolytically-Resistant Boron-Containing Therapeutics And Methods Of Use | Anacor Pharmaceuticals | 2-
May- 06 |
2-
May -07 |
15-
Nov -07 |
| US20070259
914 |
Novel Piperidine Derivates as Modulators of Chemokine Receptor Ccr5. | TUCKER HOWARD | 24-
Jun- 04 |
20-
Jun -05 |
8-
Nov -07 |
| US20070259
907 |
ARYL AND ARYLALKYLENYL SUBSTITUTED THIAZOLOQUINOLINES
AND THIAZOLONAPHTHYRIDINES |
PRINCE RYAN B | 18-
Jun- 04 |
17-
Jun -05 |
8-
Nov -07 |
| US20070259
881 |
Substituted Imidazo Ring Systems and Methods | CELEBI AZIM A | 18-
Jun- 04 |
17-
Jun -05 |
8-
Nov -07 |
| US20070258
999 |
Sars Virus Nucleotide and Amino Acid Sequences and Uses Thereof | THE PUBLIC HEALTH AGENCY OF CANADA | 28-
Apr- 03 |
28-
Apr -04 |
8-
Nov -07 |
| US20070254
906 |
Method of Administration of Dopamine Receptor Agonists | DarPharma, Inc. | 21-
Jul-04 |
21-
Jul- 05 |
1-
Nov -07 |
| US20070254
329 |
Mammalian Genes Involved in Viral Infection and Tumor Suppression | RUBIN DONALD H | 2-
May- 02 |
2-
May -03 |
1-
Nov -07 |
| US20070253
978 |
Copy choice recombination and uses thereof | NIMAN HENRY L | 2-Jul-
04 |
29-
Dec -06 |
1-
Nov -07 |
| US20070253
860 |
Process and device for sterilising ambient air | SCHRODER WERNER | 18-
Oct- 04 |
18-
Apr -07 |
1-
Nov -07 |
| US20070249
686 |
Modulators of Crth-2 Receptor Activity for the Treatment of Prostaglandin D2 Mediated Diseases | ASTRAZENECA AB | 5-
Oct- 04 |
3-
Oct -05 |
25-
Oct- 07 |
| US20070248
969 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF BACTERIA | BLYN LAWRENCE | 11-
Sep- 03 |
13-
Mar -07 |
25-
Oct- 07 |
| US20070248
949 |
Sensitive and Specific Test to Detect Sars Coronavirus | AGENCY FOR SCIENCE, TECHNOLOGY AND RESEARCH | 17-
Dec- 03 |
17-
Dec -04 |
25-
Oct- 07 |
| US20070244
133 |
Thienopyrimidines and Thiazolopyrimidines for Use in Medicine | BOWER JUSTIN F | 4-
Jun- 04 |
31-
May -05 |
18-
Oct- 07 |
| US20070244
044 |
Antimicrobial Peptides | NOVABIOTICS LIMITED | 18-
Aug- 04 |
18-
Aug -05 |
18-
Oct- 07 |
| US20070243
600 |
System for performing multi-formatted assays | GEN-PROBE INCORPORATED | 10-
Mar- 05 |
10-
Mar -06 |
18-
Oct- 07 |
| US20070243
544 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF BACTERIA | BLYN LAWRENCE | 11-
Sep- 03 |
13-
Mar -07 |
18-
Oct- 07 |
| US20070243
263 |
Antiviral Methods | AgION Technologies, Inc. | 14-
Apr- 06 |
21-
Mar -07 |
18-
Oct- 07 |
| US20070238
681 |
MODULATION OF ACE2 EXPRESSION | BENNETT C F | 7-
Nov- 06 |
7-
Dec -06 |
11-
Oct- 07 |
| US20070238
116 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF BACTERIA | BLYN LAWRENCE | 11-
Sep- 03 |
13-
Mar -07 |
11-
Oct- 07 |
| US20070231
347 |
Immunization Regimen with E4-Deleted Adenovirus Prime and E1- Deleted Adenovirus Boost | The Trustees of the University of Pennslyvania | 28-
Apr- 04 |
27-
Apr -05 |
4-
Oct- 07 |
| US20070231
344 |
Conjugate vaccines for non-proteinaceous antigens | The Brigham and Women’s Hospital, Inc. | 28-
Oct- 05 |
30-
Oct -06 |
4-
Oct- 07 |
| US20070231
303 |
METHODS OF GENERATING CHIMERIC ADENOVIRUSES AND USES FOR SUCH CHIMERIC ADENOVIRUSES | The Trustees of the University of Pennsylvania | 20-
Jun- 03 |
20-
Jun -03 |
4-
Oct- 07 |
| US20070231
295 |
Antimicrobial Silicon Oxide Flakes | BUJARD PATRICE | 12-
May- 04 |
2-
May -05 |
4-
Oct- 07 |
| US20070225
303 |
8-Oxoadenine Compound | HASHIMOTO KAZUKI | 26-
Mar- 04 |
25-
Mar -05 |
27-
Sep -07 |
| US20070225
205 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | MAXYGEN, INC. | 19-
May- 04 |
14-
Sep -06 |
27-
Sep -07 |
| US20070225
204 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | MAXYGEN, INC. | 19-
May- 04 |
14-
Sep -06 |
27-
Sep -07 |
| US20070224
700 |
Method and apparatus for analyzing bioprocess fluids | BioScale, Inc. | 2-
May- 05 |
19-
Dec -06 |
27-
Sep -07 |
| US20070224
614 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF BACTERIA | BLYN LAWRENCE | 11-
Sep- 03 |
7-
Mar -07 |
27-
Sep -07 |
| US20070219
228 |
Aryl substituted imidazonaphthyridines | AMOS DAVID T | 18-
Jun- 04 |
17-
Jun -05 |
20-
Sep -07 |
| US20070219
211 |
Bicyclic Heterocyclic Derivatives and Their Use as Inhibitors of Stearoyl-Coadesaturase (Scd) | XENON PHARMACEUTICALS INC. | 20-
Sep- 04 |
20-
Sep -05 |
20-
Sep -07 |
| US20070219
200 |
PRODRUGS OF HETEROARYL COMPOUNDS | Koronos Pharmaceuticals, Incorporated | 20-
Jun- 03 |
15-
May -07 |
20-
Sep -07 |
| US20070219
196 |
AMIDE SUBSTITUTED IMIDAZOPYRIDINES, IMIDAZOQUINOLINES, AND IMIDAZONAPHTHYRIDINES | AMOS DAVID T | 24-
Mar- 04 |
24-
Mar -05 |
20-
Sep -07 |
| US20070219
149 |
Novel Vaccine Containing Adjuvant Capable Of Inducing Mucosal Immunity | Japan as Represented by the Director-General of National Institute of Infectious Diseases | 11-
Aug- 03 |
10-
Aug -04 |
20-
Sep -07 |
| US20070218
536 |
Polyvalent Viral Vectors and a System for Production Thereof | GAO GUANGPING | 28-
Apr- 04 |
27-
Apr -05 |
20-
Sep -07 |
| US20070218
489 |
COMPOSITIONS FOR USE IN IDENTIFICATION OF BACTERIA | BLYN LAWRENCE | 11-
Sep- 03 |
13-
Mar -07 |
20-
Sep -07 |
| US20070218
467 |
Methods for rapid identification and quantitation of nucleic acid variants | ECKER DAVID J | 21-
Jul-05 |
21-
Jul- 06 |
20-
Sep -07 |
| US20070218
001 |
Biotherapeutics, Diagnostics and Research Reagents | BIOTECH STUDIO,LLC | 5-
Jan- 04 |
4-
Jan -05 |
20-
Sep -07 |
| US20070213
356 |
Nitrogen-Containing Heterocyclyl Substituted Imidazoquinolines and Imidazonaphthyridines | HARALDSON CHAD A | 15-
Jun- 04 |
15-
Jun -05 |
13-
Sep -07 |
| US20070213
309 |
Sepsis Treatment Methods | AHLEM CLARENCE N | 28-
Aug- 02 |
16-
Oct -06 |
13-
Sep -07 |
| US20070212
770 |
Oligopeptide-free cell culture media | Baxter Healthcare, S.A. | 4-
Jan- 06 |
3-
Jan -07 |
13-
Sep -07 |
| US20070212
677 |
Identifying off-target effects and hidden phenotypes of drugs in human cells | Odyssey Thera, Inc. | 22-
Nov- 04 |
31-
Aug -06 |
13-
Sep -07 |
| US20070208
052 |
Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines | HEPPNER PHILIP D | 18-
Jun- 04 |
17-
Jun -05 |
6-
Sep -07 |
| US20070207
526 |
Norovirus and sapovirus antigens | COIT DORIS | 22-
Nov- 05 |
22-
Nov -06 |
6-
Sep -07 |
| US20070203
209 |
Useful indole compounds | BARTOLINI WILMIN | 18-
Aug- 05 |
18-
Aug -06 |
30-
Aug -07 |
| US20070203
107 |
Pharmaceutical compositions | Hollis-Eden Pharmaceuticals, Inc. | 28-
Aug- 02 |
13-
Oct -06 |
30-
Aug -07 |
| US20070203
082 |
RNAI Agents For Anti-SARS Coronavirus Therapy | Intradigm Corporation | 25-
Apr- 03 |
26-
Apr -04 |
30-
Aug -07 |
| US20070203
073 |
SARS and Ebola inhibitors and use thereof, and methods for their discovery | BATES PAUL | 22-
Jun- 05 |
21-
Jun -06 |
30-
Aug -07 |
| US20070203
060 |
Casein Derived Peptides And Therapeutic Uses Thereof | SIDELMAN ZVI | 1-
Mar- 04 |
1-
Sep -06 |
30-
Aug -07 |
| US20070202
492 |
Viral Assay | University Of Warwick | 15-
Apr- 04 |
14-
Apr -05 |
30-
Aug -07 |
| US20070197
646 |
SUBSTITUTED TARAXASTANES USEFUL FOR TREATING VIRAL INFECTIONS | ACHILLION PHARMACEUTICALS, INC. | 21-
Feb- 06 |
20-
Feb -07 |
23-
Aug -07 |
| US20070197
478 |
NOVEL PHARMACEUTICALS | PFIZER LTD | 17-
Feb- 06 |
16-
Feb -07 |
23-
Aug -07 |
| US20070196
818 |
Using Nucleic Acids for Clinical Microbiology Testing | O’HARA STEPHEN | 22-
Oct- 03 |
22-
Oct -04 |
23-
Aug -07 |
| US20070196
434 |
Methods of preventing or treating sinusitis with oxidative reductive potential water solution | Oculus Innovative Sciences, Inc. | 20-
Jan- 06 |
22-
Jan -07 |
23-
Aug -07 |
| US20070196
357 |
Methods of treating or preventing inflammation and hypersensitivity with oxidative reductive potential water solution | Oculus Innovative Sciences, Inc. | 20-
Jan- 06 |
22-
Jan -07 |
23-
Aug -07 |
| US20070196
333 |
Composition comprising mixtures of IFN-alpha subtypes | HUANG SHIR-LY | 23-
Feb- 06 |
23-
Feb -06 |
23-
Aug -07 |
| US20070196
274 |
Immunoconjugates with improved efficacy for the treatment of diseases | SUN LE | 20-
Jan- 06 |
19-
Jan -07 |
23-
Aug -07 |
| US20070192
906 |
Rice plant having vaccine gene transferred thereinto | EBINUMA HIROYASU | 9-
Apr- 04 |
8-
Apr -05 |
16-
Aug -07 |
| US20070192
905 |
Edible vaccines expressed in soybeans | The University of North Carolina | 12-
Oct- 04 |
12-
Oct -05 |
16-
Aug -07 |
| US20070191
294 |
Short interfering rna (sirna) analogues | SANTARIS PHARMA AS | 21-
Mar- 03 |
22-
Mar -04 |
16-
Aug -07 |
| US20070190
163 |
Technology for preparation of macromolecular microspheres | FANG FANG | 24-
Jan- 06 |
24-
Jan -07 |
16-
Aug -07 |
| US20070190
071 |
9-Substituted 8-oxoadenine compound | AstraZeneca Aktiebolag | 26-
Mar- 04 |
24-
Mar -05 |
16-
Aug -07 |
| US20070190
065 |
Nucleic acids, polypeptides, methods of expression, and immunogenic compositions associated with SARS corona virus spike protein | ALTMEYER RALF | 3-
Jun- 05 |
4-
Dec -06 |
16-
Aug -07 |
| US20070190
031 |
Plasmid having three complete transcriptional units and immunogenic compositions for inducing an immune response to hiv | EGAN MICHAEL | 17-
Jun- 04 |
15-
Jun -05 |
16-
Aug -07 |
| US20070185
044 |
Modulation of ace2 expression | BENNETT C F | 8-
Mar- 05 |
8-
Mar -05 |
9-
Aug -07 |
| US20070185
027 |
ANTIVIRAL AGENTS FOR THE TREATMENT, CONTROL AND PREVENTION OF INFECTIONS BY CORONAVIRUSES | SEQUOIA PHARMACEUTICALS, INC. | 28-
Apr- 03 |
9-
Nov -06 |
9-
Aug -07 |
| US20070184
434 |
Compositions for use in identification of influenza viruses | ESHOO MARK W | 17-
Oct- 05 |
17-
Oct -06 |
9-
Aug -07 |
| US20070178
533 |
Method and diagnostic tests based on flow cytometric analysis of antigen-specific t lymphocytes | AGRATI CHIARA | 5-
Aug- 03 |
5-
Aug -04 |
2-
Aug -07 |
| US20070178
505 |
Promoter engineering and genetic control | ALPER HAL S | 3-
Jan- 06 |
3-
Jan -07 |
2-
Aug -07 |
| US20070178
048 |
Antiviral composition comprising p-menthane-3,8-diol | CLARKE PAUL D | 12-
Mar- 04 |
2-
Mar -05 |
2-
Aug -07 |
| US20070173
755 |
Methods of treating or preventing peritonitis with oxidative reductive potential water solution | Oculus Innovative Sciences, Inc. | 20-
Jan- 06 |
22-
Jan -07 |
26-
Jul- 07 |
| US20070173
481 |
Compositions and methods for preventing infection | JOLLA BIOSCIENCES LLC | 20-
Sep- 02 |
11-
Dec -06 |
26-
Jul- 07 |
| US20070172
817 |
Methods of Producing Antibodies for Diagnostics and Therapeutics | CHANG XIAO-JIA | 8-Jul-
03 |
22-
Nov -06 |
26-
Jul- 07 |
| US20070172
448 |
Method for detecting the specificity of activated lymphocyte | HU JUN | 8-
Dec- 03 |
7-
Dec -04 |
26-
Jul- 07 |
| US20070167
476 |
Piperazine, [1,4]Diazepane, [1,4]Diazocane, and [1,5]Diazocane fused imidazo ring compounds | CELEBI AZIM A | 29-
Dec- 03 |
22-
Dec -04 |
19-
Jul- 07 |
| US20070167
408 |
NOVEL ALKYL PHOSPHOLIPID DERIVATIVES WITH REDUCED
CYTOTOXICITY AND USES THEREOF |
ZENTARIS GmbH | 19-
Dec- 05 |
19-
Dec -06 |
19-
Jul- 07 |
| US20070166
823 |
Methods for detecting parvovirous infections | BLUTH MARTIN H | 5-
May- 03 |
5-
May -04 |
19-
Jul- 07 |
| US20070166
784 |
Combination approaches for generating immune responses | BARNETT SUSAN W | 15-
Sep- 03 |
15-
Sep -04 |
19-
Jul- 07 |
| US20070166
384 |
Methods , composition and preparations for delivery of immune response modifiers | ZARRAGA ISIDRO ANGELO E | 9-
Apr- 04 |
8-
Apr -05 |
19-
Jul- 07 |
| US20070166
281 |
Chloroquine coupled antibodies and other proteins with methods for their synthesis | KOSAK KENNETH M | 21-
Aug- 04 |
22-
Feb -07 |
19-
Jul- 07 |
| US20070160
981 |
VIRAL PROTEASE | CHEN XIN | 24-
Oct- 05 |
23-
Oct -06 |
12-
Jul- 07 |
| US20070155
767 |
Sulfone substituted imidazo ring ethers | DELLARIA JOSEPH F JR | 4-
Dec- 03 |
3-
Dec -04 |
5-
Jul- 07 |
| US20070155
699 |
Boron-containing small molecules | Anacor Pharmaceuticals | 30-
Dec- 05 |
16-
Aug -06 |
5-
Jul- 07 |
| US20070155
683 |
Artificial cpg single-stranded oligodeoxynucleotide and antiviral use thereof | WANG LIYING | 25-
Jul-03 |
26-
Jul- 04 |
5-
Jul- 07 |
| US20070154
348 |
Supports for assaying analytes and methods of making and using thereof | FRUTOS ANTHONY G | 29-
Dec- 05 |
7-
Jun -06 |
5-
Jul- 07 |
| US20070149
487 |
Antiviral Compositions and Methods | MAYO FOUNDATION FOR MEDICAL EDUCATION AND RESEARCH | 8-
Nov- 05 |
7-
Nov -06 |
28-
Jun- 07 |
| US20070148
670 |
Methods for detecting conformational changes in bioentities | O’MALLEY SHAWN M | 28-
Dec- 05 |
25-
Oct -06 |
28-
Jun- 07 |
| US20070141
597 |
Biomimetic Biodetector of Toxins, Viruses, Bacteria, and Biological Factors | HARMON H J | 25-
Oct- 05 |
25-
Oct -06 |
21-
Jun- 07 |
| US20070141
080 |
Treating severe acute respiratory syndrome | HEMISPHERx BIOPHARMA | 16-
May- 03 |
26-
Jan -07 |
21-
Jun- 07 |
| US20070141
053 |
Treatment of inflammatory respiratory diseases | Schering Aktiengesellschaft | 9-
May- 03 |
7-
May -04 |
21-
Jun- 07 |
| US20070136
890 |
Expression of a recombinant transgene | Board of Trustees Operating Michigan State University | 3-Jul-
03 |
2-
Jul- 04 |
14-
Jun- 07 |
| US20070135
439 |
Novel inhibitors of cysteine proteases, the pharmaceutical compositions thereof and their therapeutic applications | BOISSY GUILLAUME | 8-
Dec- 05 |
8-
Dec -05 |
14-
Jun- 07 |
| US20070135
367 |
Method for controlling sr protein phosphorylation, and antiviral agents whose active ingredients comprise agents that control sr protein activity | FUKUHARA TAKESHI | 26-
Dec- 03 |
24-
Dec -04 |
14-
Jun- 07 |
| US20070134
695 |
Dengue virus detection measured by immunocytometry in a dendritic cell surrogate | BURGESS TIMOTHY | 27-
Oct- 05 |
26-
Oct -06 |
14-
Jun- 07 |
| US20070134
694 |
Method for the measurement of dengue virus binding inhibition | BURGESS TIMOTHY | 27-
Oct- 05 |
26-
Oct -06 |
14-
Jun- 07 |
| US20070134
255 |
Hmpv treatment with ribavirin and anti-hmpv antibody | ViroNovative B.V. | 2-
May- 03 |
3-
May -04 |
14-
Jun- 07 |
| US20070134
214 |
Inactivated host cell delivery of polynucleotides encoding immunogens | XU FENG | 13-
Aug- 03 |
12-
Aug -04 |
14-
Jun- 07 |
| US20070123
566 |
Isoflavone derivatives of tectoridin, the preparation thereof and the anti-virus medicines containing the same as an effective constituents | CHENGDU DIKANG PHARMACEUTICAL INSTITUTE | 15-
May- 03 |
14-
May -04 |
31-
May -07 |
| US20070116
785 |
Nitric oxide as an anti-viral agent, vaccine and vaccine adjuvant | MILLER CHRISTOPHER C | 18-
Nov- 05 |
20-
Nov -06 |
24-
May -07 |
| US20070116
716 |
Sars coronavirus s proteins and uses thereof | FIELDING BURTRAM C | 10-
Dec- 03 |
10-
Dec -04 |
24-
May -07 |
| US20070116
600 |
Detection device and methods associated therewith | COOK RICHARD A | 23-
Jun- 05 |
23-
Jun -06 |
24-
May -07 |
| US20070111
309 |
Vero cell line adapted to grow in suspension | DAELLI MARCELO G | 4-
Oct- 05 |
29-
Sep -06 |
17-
May -07 |
| US20070111
296 |
Methods and Devices for Quantitative Viral Assays | SHU YING | 16-
Nov- 05 |
16-
Nov -06 |
17-
May -07 |
| US20070111
231 |
Detection of mutations in a gene associated with resistance to viral infection, OAS2 and OAS3 | Illumigen Biosciences, Inc. | 23-
Aug- 05 |
23-
Aug -06 |
17-
May -07 |
| US20070105
193 |
Severe acute respiratory syndrome DNA vaccine compositions and methods of use | Vical Incorporated | 16-
May- 03 |
12-
May -04 |
10-
May -07 |
| US20070104
808 |
COMPOSITIONS AND METHODS FOR TREATMENT OF RHINOVIRUS | THE QUIGLEY CORPORATION | 6-
Aug- 01 |
27-
Oct -06 |
10-
May -07 |
| US20070104
686 |
Vaccines, immunotherapeutics and methods for using the same | CHATTERGOON MICHAEL A | 13-
Jun- 03 |
14-
Jun -04 |
10-
May -07 |
| US20070099
968 |
Antiviral compounds and methods | Biotron Limited | 24-
Jun- 04 |
24-
Jun -04 |
3-
May -07 |
| US20070099
901 |
Hydroxylamine and oxime substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines | 3M INNOVATIVE PROPERTIES COMPANY | 25-
Nov- 03 |
24-
Nov -04 |
3-
May -07 |
| US20070099
855 |
Glycyrrhizin or derivatives thereof for for treating or preventing severe acute respiratory syndrome (sars) | Johann Wolfgang Goethe University | 6-
Jun- 03 |
7-
Jun -04 |
3-
May -07 |
| US20070099
178 |
Method for detecting sars coronavirus | EIKEN KAGAKU KABUSHIKI KAISHA | 27-
Jun- 03 |
15-
Jun -04 |
3-
May -07 |
| US20070098
735 |
Methods for the Elimination of Pathogens and Other Particulate Agents | CHANDAWARKAR RAJIV Y | 29-
Oct- 05 |
30-
Oct -06 |
3-
May -07 |
| US20070098
719 |
GITR binding molecules and uses therefor | TolerRx, Inc. | 25-
Mar- 05 |
27-
Mar -06 |
3-
May -07 |
| US20070093
969 |
Molecular nephrotoxicology modeling | CASTLE ARTHUR | 22-
Nov- 02 |
24-
Nov -03 |
26-
Apr- 07 |
| US20070092
938 |
Diagnostics for sars virus | Temasek Life Sciences Laboratory | 15-
Jul-03 |
4-
Feb -04 |
26-
Apr- 07 |
| US20070092
936 |
Severe acute respiratory syndrome | HAYNES BARTON F | 8-
May- 03 |
10-
May -04 |
26-
Apr- 07 |
| US20070092
871 |
Microarray for pathogen identification | COMBIMATRIX CORP | 20-
Oct- 05 |
20-
Oct -06 |
26-
Apr- 07 |
| US20070087
974 |
TREATMENT OR PREVENTION OF RESPIRATORY VIRAL INFECTIONS WITH IMMUNOMODULATOR COMPOUNDS | SCICLONE PHARMACEUTICALS, INC. | 14-
May- 04 |
9-
Nov -06 |
19-
Apr- 07 |
| US20070087
341 |
Compositions for use in identification of influenza viruses | ESHOO MARK W | 17-
Oct- 05 |
17-
Oct -06 |
19-
Apr- 07 |
| US20070087
340 |
Compositions for use in identification of influenza viruses | ESHOO MARK W | 17-
Oct- 05 |
17-
Oct -06 |
19-
Apr- 07 |
| US20070087
339 |
Compositions for use in identification of influenza viruses | ESHOO MARK W | 17-
Oct- 05 |
17-
Oct -06 |
19-
Apr- 07 |
| US20070087
338 |
Compositions for use in identification of influenza viruses | ESHOO MARK W | 17-
Oct- 05 |
17-
Oct -06 |
19-
Apr- 07 |
| US20070087
337 |
Compositions for use in identification of influenza viruses | ESHOO MARK W | 17-
Oct- 05 |
17-
Oct -06 |
19-
Apr- 07 |
| US20070087
336 |
Compositions for use in identification of influenza viruses | ESHOO MARK W | 17-
Oct- 05 |
17-
Oct -06 |
19-
Apr- 07 |
| US20070087
332 |
Pulmonary stem cells, related methods and kits of parts | LING THAI-YEN | 17-
Oct- 05 |
17-
Oct -05 |
19-
Apr- 07 |
| US20070087
008 |
Rab9a, rablla, and modulators thereof related to infectious disease | UNIV VANDERBILT | 24-
Feb- 04 |
24-
Feb -05 |
19-
Apr- 07 |
| US20070082
971 |
Use of a plastic composition and a product obtained thereby | POLYGIENE AB | 23-
Feb- 04 |
27-
Jan -05 |
12-
Apr- 07 |
| US20070077
201 |
Stem cell expansion and uses | DOWDING CHARLES | 29-
Sep- 04 |
25-
Mar -06 |
5-
Apr- 07 |
| US20070072
893 |
Substituted imidazo ring systems and methods | AMOS DAVID T | 25-
Nov- 03 |
24-
Nov -04 |
29-
Mar -07 |
| US20070072
202 |
Use of chimeric receptors in a screening assay for identifying agonists and antagonists of cell receptors | BATES ELIZABETH E M | 24-
Mar- 05 |
24-
Mar -06 |
29-
Mar -07 |
| US20070066
649 |
METHOD OF TREATING OR INHIBITING THE DEVELOPMENT OF BRAIN INFLAMMATION AND SEPSIS | Not Available | 29-
Nov- 02 |
1-
Nov -06 |
22-
Mar -07 |
| US20070066
648 |
METHOD OF TREATING OR INHIBITING THE DEVELOPMENT OF BRAIN INFLAMMATION AND SEPSIS | NOZAKI MASAKO | 29-
Nov- 02 |
1-
Nov -06 |
22-
Mar -07 |
| US20070066
639 |
Oxime substituted imidazoquinolines | AMOS DAVID T | 12-
Aug- 04 |
12-
Aug -04 |
22-
Mar -07 |
| US20070066
552 |
Topical administration permitting prolonged exposure of target cells to therapeutic and prophylactic nucleic acids | INTROGEN THERAPEUTICS INC | 21-
Jan- 05 |
20-
Jan -06 |
22-
Mar -07 |
| US20070065
939 |
Systems for detection and production of respiratory, herpes and enteric viruses | Diagnostic Hybrids, Inc. | 20-
Sep- 05 |
28-
Apr -06 |
22-
Mar -07 |
| US20070065
407 |
Interferon-Alpha Polypeptides and Conjugates | MAXYGEN INC | 18-
Nov- 02 |
30-
Oct -06 |
22-
Mar -07 |
| US20070060
754 |
Alkoxy substituted imidazoquinolines | HARALDSON CHAD A | 3-
Oct- 03 |
1-
Oct -04 |
15-
Mar -07 |
| US20070060
611 |
METHOD OF TREATING OR INHIBITING THE DEVELOPMENT OF BRAIN INFLAMMATION AND SEPSIS | Not Available | 29-
Nov- 02 |
1-
Nov -06 |
15-
Mar -07 |
| US20070060
535 |
Targeted delivery of antiviral compounds through hemoglobin bioconjugates | ADAMSON J G | 30-
Apr- 98 |
13-
Nov -06 |
15-
Mar -07 |
| US20070060
499 |
Chloroquine combination drugs and methods for their synthesis | KOSAK KENNETH M | 15-
Sep- 05 |
22-
Feb -06 |
15-
Mar -07 |
| US20070059
728 |
COMPUTER-IMPLEMENTED BIOLOGICAL SEQUENCE IDENTIFIER SYSTEM AND METHOD | The Government of the US, as represented by the Secretary of the Navy | 2-Jul-
04 |
6-
Jun -06 |
15-
Mar -07 |
| US20070059
686 |
Materials and methods for the detection of severe acute respiratory syndrome virus (SARS) | ERAGEN BIOSCIENCES INC | 31-
Jan- 04 |
28-
Jul- 06 |
15-
Mar -07 |
| US20070059
243 |
METHOD OF TREATING OR INHIBITING THE DEVELOPMENT OF BRAIN INFLAMMATION AND SEPSIS | Not Available | 29-
Nov- 02 |
1-
Nov -06 |
15-
Mar -07 |
| US20070054
937 |
METHOD OF TREATING OR INHIBITING THE DEVELOPMENT OF BRAIN INFLAMMATION AND SEPSIS | Not Available | 29-
Nov- 02 |
1-
Nov -06 |
8-
Mar -07 |
| US20070054
834 |
Quaternary ammonium halides for treatment of infectious conditions | NANOBIO CORP | 11-
Apr- 05 |
10-
Apr -06 |
8-
Mar -07 |
| US20070053
933 |
IL28 and IL29 TRUNCATED CYSTEINE MUTANTS AND ANTIVIRAL METHODS OF USING SAME | SHEPPARD PAUL O | 20-
Jul-05 |
20-
Jul- 06 |
8-
Mar -07 |
| US20070053
920 |
Nematode polypeptide adjuvant | Not Available | 22-
Jan- 03 |
21-
Jan -04 |
8-
Mar -07 |
| US20070053
878 |
Sars | FOUCHIER RONALDUS A M | 8-
Apr- 03 |
8-
Apr -04 |
8-
Mar -07 |
| US20070048
759 |
DETECTION OF TARGET MOLECULES WITH LABELED NUCLEIC ACID DETECTION MOLECULES | LI YOUGEN | 10-
Jun- 05 |
12-
Jun -06 |
1-
Mar -07 |
| US20070048
747 |
Methods for assaying analytes | LESLIE THOMAS M | 1-
Sep- 05 |
1-
Sep -05 |
1-
Mar -07 |
| US20070048
282 |
Albumin fusion proteins | Human Genome Sciences, Inc. | 9-
Feb- 04 |
8-
Aug -06 |
1-
Mar -07 |
| US20070042
441 |
Method and apparatus for detecting estradiol and metabolites thereof using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
22-
Feb -07 |
| US20070042
388 |
Method of probe design and/or of nucleic acids detection | LEE CHARLIE | 12-
Aug- 05 |
12-
Aug -05 |
22-
Feb -07 |
| US20070042
381 |
BIOINFORMATICALLY DETECTABLE GROUP OF NOVEL REGULATORY VIRAL AND VIRAL ASSOCIATED OLIGONUCLEOTIDES AND USES THEREOF | Rosetta Genomics | 16-
Jan- 03 |
26-
May -04 |
22-
Feb -07 |
| US20070042
351 |
Multi-allelic molecular detection of sars-associated coronavirus | KOSTRIKIS LEONDIOS G | 22-
Aug- 03 |
13-
Aug -04 |
22-
Feb -07 |
| US20070042
350 |
Methods and compositions for detecting sars virus and other infectious agents | CHENG JING | 14-
Jul-03 |
14-
Jul- 03 |
22-
Feb -07 |
| US20070041
941 |
Nucleic acid sequences encoding and compositions comrpising ige signal peptide and/or il-15 and methods for using the same | BOYER JEAN D | 13-
Jun- 03 |
14-
Jun -04 |
22-
Feb -07 |
| US20070037
763 |
Oligonucleotide compound and method for treating nidovirus infections | AVI BIOPHARMA INC | 24-
Dec- 03 |
10-
May -06 |
15-
Feb -07 |
| US20070037
231 |
Methods and apparatus for detecting bacteria using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
15-
Feb -07 |
| US20070037
142 |
Methods and apparatus for detecting viruses using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
15-
Feb -07 |
| US20070037
140 |
Methods and compositions for detecting sars virus | CAPITAL BIOCHIP COMPANY, LTD. | 9-
May- 03 |
9-
May -03 |
15-
Feb -07 |
| US20070036
760 |
Adeno-associated virus (aav) clades, sequences, vectors containing same, and uses therefor | The Trutees of the University of Pennsylvania | 30-
Sep- 03 |
30-
Sep -04 |
15-
Feb -07 |
| US20070036
744 |
Treatment or prevention of respiratory viral infections with alpha thymosin peptides | SciClone Pharmaceuticals, Inc. | 23-
Apr- 03 |
23-
Apr -04 |
15-
Feb -07 |
| US20070034
529 |
Electrochemistry and electrogenerated chemiluminescence with a single faradaic electrode | BARD ALLEN J | 3-
Jun- 05 |
2-
Jun -06 |
15-
Feb -07 |
| US20070032
499 |
Novel cysteine protease inhibitors and their therapeutic applications | BOISSY GUILLAUME | 5-
Aug- 05 |
5-
Aug -05 |
8-
Feb -07 |
| US20070031
923 |
Modified viral particles with immunogenic properties and reduced lipid content useful for treating and preventing infectious diseases | CHAM BILL E | 29-
Jun- 00 |
10-
Apr -06 |
8-
Feb -07 |
| US20070031
832 |
Methods of constructing biodiverse gene fragment libraries and biological modulators isolated therefrom | Phylogica Limited | 5-
May- 99 |
20-
Feb -04 |
8-
Feb -07 |
| US20070031
283 |
Assay cartridges and methods for point of care instruments | BLANKFARD MARTIN | 23-
Jun- 05 |
23-
Jun -06 |
8-
Feb -07 |
| US20070027
306 |
Albumin fusion proteins | HASELTINE WILLIAM A | 9-
Feb- 04 |
8-
Aug -06 |
1-
Feb -07 |
| US20070026
391 |
Methods and compositions for identifying chemical or biological agents using multiplexed labeling and colocalization detection | GHC TECHNOLOGIES INC | 11-
Apr- 05 |
6-
Apr -06 |
1-
Feb -07 |
| US20070026
087 |
Anti-coronavirus agent | Toagosei Co., Ltd. | 16-
Oct- 03 |
31-
Oct -03 |
1-
Feb -07 |
| US20070026
014 |
Interferon beta in severe acute respiratory syndrome (sars) | ARES TRADING S.A. | 17-
Apr- 03 |
6-
Apr -04 |
1-
Feb -07 |
| US20070026
009 |
Systems and methods for identifying replikin scaffolds and uses of said replikin scaffolds | BOGOCH ELENORE S | 27-
Mar- 01 |
16-
Feb -06 |
1-
Feb -07 |
| US20070025
966 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | MAXYGEN, INC. | 19-
May- 04 |
13-
Sep -06 |
1-
Feb -07 |
| US20070021
439 |
Methods of reducing risk of infection from pathogens with soluble amide and ester pyrazinoylguanidine sodium channel blockers | PARION SCIENCES INC | 25-
Jul-05 |
25-
Jul- 05 |
25-
Jan- 07 |
| US20070021
326 |
Composition and its Therapeutic Use | INSIGNION HOLDINGS LTD AND VER | 9-
Aug- 02 |
25-
Sep -06 |
25-
Jan- 07 |
| US20070020
734 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | MAXYGEN, INC. | 19-
May- 04 |
13-
Sep -06 |
25-
Jan- 07 |
| US20070020
235 |
INTERFERON-ALPHA POLYPEPTIDES AND CONJUGATES | MAXYGEN, INC. | 19-
May- 04 |
13-
Sep -06 |
25-
Jan- 07 |
| US20070015
181 |
System and methods for nucleic acid and polypeptide selection | WILLIAMS RICHARD B | 19-
May- 00 |
2-
May -06 |
18-
Jan- 07 |
| US20070015
172 |
Expression profiles for microbial infection | Z-BioMed, Inc. | 1-
Jun- 05 |
30-
Nov -05 |
18-
Jan- 07 |
| US20070014
778 |
Endoribonuclease and uses thereof | LEE CHOW | 6-
Jun- 05 |
5-
Jun -06 |
18-
Jan- 07 |
| US20070014
719 |
Steroid analogs and characterization and treatment methods | DOWDING CHARLES | 29-
Sep- 04 |
29-
Sep -05 |
18-
Jan- 07 |
| US20070009
932 |
Promoter engineering and genetic control | ALPER HAL S | 27-
Apr- 05 |
26-
Apr -06 |
11-
Jan- 07 |
| US20070009
884 |
Methods and apparatuses for detecting chemical or biological agents | GHC TECHNOLOGIES INC | 11-
Apr- 05 |
6-
Apr -06 |
11-
Jan- 07 |
| US20070004
028 |
Signal measuring system for conducting real-time amplification assays | GEN-PROBE INCORPORATED | 10-
Mar- 05 |
10-
Mar -06 |
4-
Jan- 07 |
| US20070003
577 |
Purified trimeric S protein as vaccine against severe acute respiratory syndrome virus infections | CHU KID | 28-
Jun- 05 |
27-
Jun -06 |
4-
Jan- 07 |
| US20070003
565 |
Use of hab18g/cd147 molecule as target for antiviral antagonists and thus obtained antiviral antagonist | CHEN ZHINAN | 9-
Jun- 03 |
9-
Jun -03 |
4-
Jan- 07 |
| US20060293
352 |
Phenoxiacetic acid derivatives | BONNERT ROGER V | 21-
Aug- 03 |
18-
Aug -04 |
28-
Dec -06 |
| US20060293
308 |
Pyridyl derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 29-
Jul-03 |
29-
Jul- 04 |
28-
Dec -06 |
| US20060293
267 |
Dual functional oligonucleotides for use as anti-viral agents | UNIVERSITY OF MASSACHUSETTS | 13-
Apr- 05 |
13-
Apr -06 |
28-
Dec -06 |
| US20060292
178 |
Proteins encoded by the severe acute respiratory syndrome (SARS) coronavirus and a role in apoptosis | Agency for Science, Technology and Research | 4-
Aug- 04 |
4-
Aug -05 |
28-
Dec -06 |
| US20060292
162 |
Plasma or serum fraction for the treatment or prevention of bacterial infections | BUCKHEIT ROBERT W JR | 22-
Apr- 05 |
24-
Apr -06 |
28-
Dec -06 |
| US20060292
151 |
L-SIGN polymorphisms and methods involving use of same | GARDNER JASON P | 26-
Mar- 04 |
28-
Mar -05 |
28-
Dec -06 |
| US20060287
263 |
Methods and compositions for inducing antigen-specific immune responses | COLEY PHARMACEUTICAL GROUP LTD | 18-
Jul-04 |
18-
Jul- 05 |
21-
Dec -06 |
| US20060286
667 |
Methods for detection and production of influenza viruses | Diagnostic Hybrids, Inc. | 24-
Apr- 98 |
28-
Apr -06 |
21-
Dec -06 |
| US20060286
124 |
Vaccine compositions and methods of treating coronavirus infection | ID Biomedical Corporation of Quebec | 30-
Jun- 04 |
30-
Jun -05 |
21-
Dec -06 |
| US20060286
121 |
Adenoviral vector-based vaccines | BROUGH DOUGLAS E | 25-
Jul-03 |
23-
Jan -06 |
21-
Dec -06 |
| US20060286
119 |
Compositions and methods for treatment of chronic and infectious diseases | Biokit S.A. | 23-
Dec- 03 |
6-
Apr -06 |
21-
Dec -06 |
| US20060281
128 |
Compositions and methods using lentivirus-based vectors for generating immune responses | VIRXSYS CORPORATION | 9-
Sep- 03 |
17-
Aug -06 |
14-
Dec -06 |
| US20060281
072 |
Agonistic Binding Molecules to the Human OX40 Receptor | BAKKER ALEXANDER BERTHOLD H | 13-
Jun- 02 |
13-
Jun -03 |
14-
Dec -06 |
| US20060280
754 |
Method of preventing virus: cell fusion by inhibiting the function of the fusion initiation region in rna viruses having class i membrane fusogenic envelope proteins | GARRY ROBERT F | 4-
Nov- 03 |
3-
Nov -04 |
14-
Dec -06 |
| US20060280
748 |
Plasma or serum fraction for treatment or prevention of abnormal cell proliferation | BUCKHEIT ROBERT W JR | 22-
Apr- 05 |
24-
Apr -06 |
14-
Dec -06 |
| US20060280
723 |
Interferon for treating or preventing a coronaviral infection | Viragen, Inc | 19-
May- 03 |
19-
May -04 |
14-
Dec -06 |
| US20060280
679 |
Method of treating autoimmune disease by inducing antigen presentation by tolerance inducing antigen presenting cells | BOWDISH KATHERINE S | 4-
Mar- 03 |
17-
Dec -04 |
14-
Dec -06 |
| US20060276
972 |
Method for determining the amount of an analyte in a sample | Gen-Probe Incorporated | 10-
Mar- 05 |
10-
Mar -06 |
7-
Dec -06 |
| US20060275
841 |
Assay method and apparatus with reduced sample matrix effects | BLANKFARD MARTIN | 20-
Dec- 04 |
19-
Dec -05 |
7-
Dec -06 |
| US20060275
802 |
Mutations in OAS1 genes | FELLIN P C | 4-
May- 05 |
3-
May -06 |
7-
Dec -06 |
| US20060275
749 |
Compositions for use in identification of orthopoxviruses | ISIS Pharmaceuticals, Inc. | 5-
Dec- 03 |
24-
Aug -05 |
7-
Dec -06 |
| US20060275
515 |
Antiviral preparations obtained from a natural cinnamon extract | OVADIA MICHAEL | 24-
Dec- 03 |
22-
Jun -06 |
7-
Dec -06 |
| US20060275
317 |
Vaccine compositions for prevention of chronic and infectious diseases | Biokit S.A. | 23-
Dec- 03 |
6-
Apr -06 |
7-
Dec -06 |
| US20060270
835 |
Diagnosis and treatment of Alzheimer disease | Biokit S.A. | 23-
Dec- 03 |
6-
Apr -06 |
30-
Nov -06 |
| US20060270
614 |
Use of chalcones for the treatment of viral disorders | BODDUPALLI SEKHAR | 24-
May- 05 |
24-
May -05 |
30-
Nov -06 |
| US20060270
041 |
Cell lines for production of replication-defective adenovirus | HOWE JOHN A | 13-
Dec- 04 |
12-
Dec -05 |
30-
Nov -06 |
| US20060270
017 |
Inactivation of a pathogen in a sample by a treatment with formalin and UV light | BARRETT NOEL | 26-
May- 05 |
26-
May -05 |
30-
Nov -06 |
| US20060269
976 |
METHOD FOR REDUCING LYSOZYME ENZYMATIC ACTIVITY | Biokit S.A. | 23-
Dec- 03 |
5-
Apr -06 |
30-
Nov -06 |
| US20060269
936 |
Screening assay for TLR7, TLR8 and TLR9 agonists and antagonists | DOLLET SANDRA | 24-
Mar- 05 |
23-
Mar -06 |
30-
Nov -06 |
| US20060269
911 |
Antisense antiviral compound and method for treating ssRNA viral infection | AVI BIOPHARMA INC | 16-
Sep- 04 |
10-
May -06 |
30-
Nov -06 |
| US20060269
572 |
Accelerated vaccination | GEISBERT THOMAS W | 1-
Aug- 03 |
17-
Jan -06 |
30-
Nov -06 |
| US20060269
538 |
Serine proteases with altered sensitivity to activity-modulating substances | COCO WAYNE M | 27-
May- 05 |
26-
May -06 |
30-
Nov -06 |
| US20060264
448 |
Purine derivatives | PFIZER LTD | 4-
May- 05 |
3-
May -06 |
23-
Nov -06 |
| US20060264
444 |
Substituted indole derivatives for pharmaceutical compositions for treating respiratory diseases | BONNERT ROGER V | 18-
Aug- 03 |
16-
Aug -04 |
23-
Nov -06 |
| US20060264
435 |
Novel compounds | BONNERT ROGER | 7-
Apr- 03 |
6-
Apr -04 |
23-
Nov -06 |
| US20060263
847 |
Compositions and methods for treatment of sever acute respiratory syndrome (sars) | SIBER GEORGE R | 20-
May- 03 |
20-
May -04 |
23-
Nov -06 |
| US20060263
765 |
Peptides and mixtures thereof for use in the detection of severe acute respiratory syndrome-associated coronavirus (sars) | HOUDE MICHEL | 9-
May- 03 |
5-
May -04 |
23-
Nov -06 |
| US20060259
249 |
Rapid identification of microbial agents | ECKER DAVID J | 3-
Mar- 04 |
3-
Mar -05 |
16-
Nov -06 |
| US20060258
611 |
Inhibition of SARS-associated coronavirus (SCoV) infection and replication by RNA interference | UNIV HONG KONG | 19-
May- 03 |
14-
Jun -06 |
16-
Nov -06 |
| US20060258
577 |
ANTIVIRAL AGENTS FOR THE TREATMENT, CONTROL AND PREVENTION OF INFECTIONS BY CORONAVIRUSES | ERICKSON JOHN W | 28-
Apr- 03 |
28-
Apr -04 |
16-
Nov -06 |
| US20060257
993 |
Integration of fluids and reagents into self-contained cartridges containing sensor elements | ANSLYN ERIC | 27-
Feb- 04 |
22-
Dec -04 |
16-
Nov -06 |
| US20060257
992 |
Integration of fluids and reagents into self-contained cartridges containing sensor elements and reagent delivery systems | ANSLYN ERIC | 27-
Feb- 04 |
22-
Dec -04 |
16-
Nov -06 |
| US20060257
991 |
Integration of fluids and reagents into self-contained cartridges containing particle-based sensor elements and membrane-based sensor elements | ANSLYN ERIC | 27-
Feb- 04 |
22-
Dec -04 |
16-
Nov -06 |
| US20060257
976 |
Methods and kits for propagating and evolving nucleic acids and proteins | RNA LINE OY | 6-
Jun- 03 |
7-
Jun -04 |
16-
Nov -06 |
| US20060257
945 |
Methods and apparatus for detecting cardiac injury markers using an acoustic device | BioScale, Inc. | 2-
May- 05 |
2-
May -06 |
16-
Nov -06 |
| US20060257
941 |
Integration of fluids and reagents into self-contained cartridges containing particle and membrane sensor elements | ANSLYN ERIC | 27-
Feb- 04 |
22-
Dec -04 |
16-
Nov -06 |
| US20060257
925 |
Method for isolating intracellular antibodies able to neutralize protein interactions | LINE GENOMICS S.P.A. | 21-
Nov- 02 |
21-
Nov -03 |
16-
Nov -06 |
| US20060257
866 |
Methods for identifying small molecules that modulate premature translation termination and nonsense mediated mrna decay | ALMSTEAD NEIL G | 24-
Jul-02 |
24-
Jul- 03 |
16-
Nov -06 |
| US20060257
861 |
Screening assay for inhibitors of severe acute respiratory syndrome (SARS) using SELDI-TOF Mass Spectrometry | Wright State University | 12-
May- 05 |
12-
May -06 |
16-
Nov -06 |
| US20060257
854 |
Membrane assay system including preloaded particles | BALLARD KARRI L | 27-
Feb- 04 |
22-
Dec -04 |
16-
Nov -06 |
| US20060257
852 |
Severe acute respiratory syndrome coronavirus | Chiron Corporation | 10-
Apr- 03 |
9-
Apr -04 |
16-
Nov -06 |
| US20060253
060 |
Method of using oxidative reductive potential water solution in dental applications | Oculus Innovative Sciences, Inc. | 2-
May- 05 |
2-
May -06 |
9-
Nov -06 |
| US20060252
767 |
Piperazine derivatives and their use as therapeutic agents | XENON PHARMACEUTICALS INC. | 30-
Jul-03 |
29-
Jul- 04 |
9-
Nov -06 |
| US20060246
081 |
Methods and compositions for polytopic vaccination | DEEM MICHAEL W | 29-
Apr- 05 |
29-
Apr -05 |
2-
Nov -06 |
| US20060241
546 |
Method of treating second and third degree burns using oxidative reductive potential water solution | Oculus Innovative Sciences, Inc. | 23-
Mar- 05 |
23-
Mar -06 |
26-
Oct- 06 |
| US20060240
551 |
Neutralizing monoclonal antibodies against severe acute respiratory syndrome-associated coronavirus | HE YUXIAN | 2-
Jun- 04 |
8-
Feb -06 |
26-
Oct- 06 |
| US20060240
530 |
Methods and compositions for infectious cDNA of SARS coronavirus | UNIV NORTH CAROLINA | 21-
Jul-03 |
19-
Jan -06 |
26-
Oct- 06 |
| US20060240
515 |
Soluble fragments of the SARS-CoV spike glycoprotein | DIMITROV DIMITER S | 21-
Jul-03 |
19-
Jan -06 |
26-
Oct- 06 |
| US20060240
412 |
Compositions for use in identification of adenoviruses | BLYN LAWRENCE | 11-
Sep- 03 |
12-
Apr -06 |
26-
Oct- 06 |
| US20060235
350 |
Method of treating skin ulcers using oxidative reductive potential water solution | Oculus Innovative Sciences, Inc. | 23-
Mar- 05 |
23-
Mar -06 |
19-
Oct- 06 |
| US20060234
981 |
Boron-containing small molecules | Anacor Pharmaceuticals | 16-
Feb- 05 |
16-
Feb -06 |
19-
Oct- 06 |
| US20060234
263 |
Method for reducing the presence of amplification inhibitors in a reaction receptacle | Gen-Probe Incorporated | 10-
Mar- 05 |
10-
Mar -06 |
19-
Oct- 06 |
| US20060229
356 |
Use of indomethacin and derivatives as broad-spectrum antiviral drugs and corresponding pharmaceutical compositions | Universita’ Degli Studi Di Roma „Tor Vergata” | 12-
Aug- 03 |
11-
Aug -04 |
12-
Oct- 06 |
| US20060228
299 |
Constructs binding to phosphatidylserine and their use in disease treatment | PEREGRINE PHARMACEUTICALS INC | 24-
Jan- 05 |
24-
Jan -06 |
12-
Oct- 06 |
| US20060223
863 |
Methods for treating Hepatitis C | AREFOLOV ALEXANDER | 14-
Jul-04 |
14-
Jul- 05 |
5-
Oct- 06 |
| US20060223
847 |
Anti-coronavirus drug | Arigen, Inc. | 15-
Jul-03 |
14-
Jul- 04 |
5-
Oct- 06 |
| US20060223
184 |
Supports useful in incorporating biomolecules into cells and methods of using thereof | FRUTOS ANTHONY G | 5-
Apr- 05 |
5-
Apr -05 |
5-
Oct- 06 |
| US20060223
074 |
Spotting compositions and methods of use thereof | BUNCH THOMAS A | 5-
Apr- 05 |
5-
Apr -05 |
5-
Oct- 06 |
| US20060216
702 |
Virus-like particles, methods of preparation, and immunogenic compositions | COMPANS RICHARD W | 17-
May- 02 |
4-
Apr -06 |
28-
Sep -06 |
| US20060211
765 |
Novel compounds | ASTRAZENECA AB | 7-
Apr- 03 |
6-
Apr -04 |
21-
Sep -06 |
| US20060211
752 |
Use of phenylmethimazoles, methimazole derivatives, and tautomeric cyclic thiones for the treatment of autoimmune/inflammatory diseases associated with toll-like receptor overexpression | BENAVIDES-PERALTA URUGUAYSITO | 16-
Mar- 04 |
17-
May -05 |
21-
Sep -06 |
| US20060211
130 |
Method for continuous mode processing of multiple reaction receptacles in a real-time amplification assay | Gen-Probe Incorporated | 10-
Mar- 05 |
10-
Mar -06 |
21-
Sep -06 |
| US20060211
115 |
Methods of generating chimeric adenoviruses and uses for such chimeric aden oviruses | The Trustees of the University of Pennsylvania | 20-
Jun- 03 |
15-
Jun -04 |
21-
Sep -06 |
| US20060210
967 |
Re-sequencing pathogen microarray | AGAN BRIAN K | 2-Jul-
04 |
2-
Jul- 05 |
21-
Sep -06 |
| US20060210
433 |
Signal measuring system having a movable signal measuring device | GEN-PROBE INCORPORATED | 10-
Mar- 05 |
10-
Mar -06 |
21-
Sep -06 |
| US20060205
713 |
Pyridazine derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 29-
Jul-04 |
29-
Jul- 04 |
14-
Sep -06 |
| US20060205
040 |
Compositions for use in identification of adventitious viruses | SAMPATH RANGARAJAN | 3-
Mar- 05 |
3-
Mar -06 |
14-
Sep -06 |
| US20060204
997 |
Method for performing multi-formatted assays | Gen-Probe Incorporated | 10-
Mar- 05 |
10-
Mar -06 |
14-
Sep -06 |
| US20060199
802 |
Pyridyl derivatives and their use as therapeutic agents | ABREO MELWYN | 30-
Jul-03 |
29-
Jul- 04 |
7-
Sep -06 |
| US20060199
176 |
Coronavirus S peptides | CHONG PELE C S | 15-
Jul-04 |
14-
Jul- 05 |
7-
Sep -06 |
| US20060195
067 |
Delivery of immune response modifier compounds | KEDL ROSS M | 25-
Aug- 03 |
25-
Aug -04 |
31-
Aug -06 |
| US20060193
745 |
Virucidal disinfectant | ARNDT ANDREAS | 28-
Jan- 05 |
27-
Jan -06 |
31-
Aug -06 |
| US20060190
189 |
Reagents, devices and methods for proteomic analysis with applications including diagnostics, vaccines, quality control and research | HOFFMANN GEOFFREY W | 21-
Apr- 04 |
16-
Mar -06 |
24-
Aug -06 |
| US20060189
644 |
Lipid-modified immune response modifiers | WIGHTMAN PAUL D | 14-
Aug- 03 |
12-
Aug -04 |
24-
Aug -06 |
| US20060189
571 |
Use of lipid conjugates in the treatment of infection | YEDGAR SAUL | 10-
Jan- 00 |
8-
Sep -05 |
24-
Aug -06 |
| US20060189
570 |
Use of lipid conjugates in the treatment of infection | YEDGAR SAUL | 10-
Jan- 00 |
8-
Sep -05 |
24-
Aug -06 |
| US20060189
569 |
Use of lipid conjugates in the treatment of infection | OJCIUS DAVID | 10-
Jan- 00 |
8-
Sep -05 |
24-
Aug -06 |
| US20060189
568 |
Use of lipid conjugates in the treatment of infection | YEDGAR SAUL | 10-
Jan- 00 |
8-
Sep -05 |
24-
Aug -06 |
| US20060189
542 |
Preventive or therapeutic composition for viral infectious disease | FURUKAWA SATORU | 22-
Jul-03 |
22-
Jul- 04 |
24-
Aug -06 |
| US20060188
519 |
Peptides, antibodies, and methods for the diagnosis of SARS | AU MUN Y D | 14-
Jun- 04 |
13-
Jun -05 |
24-
Aug -06 |
| US20060178
341 |
Composition comprising soluble glucan oligomer from saccharomyces cerevisiae is2 inhibiting the swine influenza (SIV) and transmissible gastroenteritis coronavirus (tgev) | CHUNG BONG H | 23-
Jun- 03 |
23-
Jun -04 |
10-
Aug -06 |
| US20060177
849 |
Nucleic acid primer set, nucleic acid probe set and method for detecting respiratory disease virus using the primer set and probe set | HUH NAM | 23-
Dec- 04 |
23-
Dec -05 |
10-
Aug -06 |
| US20060177
837 |
Systems and methods for identifying diagnostic indicators | BOROZAN IVAN | 13-
Aug- 04 |
15-
Aug -05 |
10-
Aug -06 |
| US20060177
813 |
Novel high-throughput screening method of drug for bioactive protein | ENDO YAETA | 8-
Sep- 03 |
8-
Sep -04 |
10-
Aug -06 |
| US20060171
962 |
SARS vaccine | Consejo Superior de Investigaciones Científicas | 3-
Sep- 04 |
2-
Sep -05 |
3-
Aug -06 |
| US20060171
958 |
Antiviral activity from medicinal mushrooms | STAMETS PAUL | 6-
Jan- 04 |
22-
Mar -06 |
3-
Aug -06 |
| US20060165
723 |
Nucleic acid sequences encoding proteins capable of associating into a virus-like particle | Fort Dodge Veterinaria S.A. | 3-
Sep- 04 |
2-
Sep -05 |
27-
Jul- 06 |
| US20060165
716 |
Immunogenic compositions for gram positive bacteria such as streptococcus agalactiae | BAROCCHI MICHELLE | 29-
Jul-04 |
29-
Jul- 05 |
27-
Jul- 06 |
| US20060163
149 |
Product for absorption purposes | HJERTEN MARIE-CHRISTINE | 13-
Jun- 03 |
11-
Jun -04 |
27-
Jul- 06 |
| US20060160
109 |
Harnessing network biology to improve drug discovery | Odyssey Thera, Inc. | 22-
Nov- 04 |
21-
Nov -05 |
20-
Jul- 06 |
| US20060154
243 |
Antigenic peptides of SARS coronavirus and uses thereof | GOUDSMIT JAAP | 21-
Jul-03 |
20-
Jan -06 |
13-
Jul- 06 |
| US20060153
803 |
Inhibition of sars coronavirus infection with clinically approved antiviral drugs | STANTON LAWRENCE W | 9-
Jun- 03 |
9-
Jun -04 |
13-
Jul- 06 |
| US20060147
958 |
System for detecting polynucleotides | CHOI K Y | 20-
May- 03 |
21-
Nov -05 |
6-
Jul- 06 |
| US20060143
719 |
Use of golden hamster as infectivity model of SARS | CONTAMIN HUGUES | 18-
Aug- 04 |
16-
Aug -05 |
29-
Jun- 06 |
| US20060142
383 |
3D-Structure model of SARS coronavirus 3CL protease and anti- SARS drugs | SHANGHAI LEAD DISCOVERY PHARMA | 4-
Jun- 03 |
2-
Dec -05 |
29-
Jun- 06 |
| US20060142
202 |
Compositions and methods for targeted delivery of immune response modifiers | 3M INNOVATIVE PROPERTIES CO | 8-
Dec- 00 |
23-
Feb -06 |
29-
Jun- 06 |
| US20060140
971 |
Cell surface expression vector of sars virus antigen and microorganisms transformed thereby | CHOI JAE C | 4-
Jun- 03 |
4-
Jun -04 |
29-
Jun- 06 |
| US20060135
458 |
Antiviral oligonucleotides | JUTEAU JEAN-MARC | 13-
Sep- 02 |
19-
Oct -05 |
22-
Jun- 06 |
| US20060135
422 |
Use of angiotensin receptor blockers (ARBs) to treat diseases associated with excess ACE | MOSKOWITZ DAVID W | 17-
Apr- 03 |
31-
Mar -04 |
22-
Jun- 06 |
| US20060134
753 |
Super-antigen fusion proteins and the use thereof | Healthbanks Biotech Co., Ltd. | 21-
Jul-04 |
19-
Jul- 05 |
22-
Jun- 06 |
| US20060134
609 |
Compositions and methods for determining the presence of SARS coronavirus in a sample | GETMAN DAMON K | 17-
Apr- 03 |
16-
Apr -04 |
22-
Jun- 06 |
| US20060134
397 |
Microporous materials, methods, and articles for localizing and quantifying analytes | SMITH ROGER E | 26-
Nov- 02 |
20-
Nov -03 |
22-
Jun- 06 |
| US20060128
628 |
Human tissue antigen-binding peptides and their amino acid sequences | CHEN SHOW-LI | 15-
Dec- 04 |
15-
Dec -04 |
15-
Jun- 06 |
| US20060123
499 |
Civet animal model system for Severe Acute Respiratory Syndrome (SARS) coronavirus infection and uses thereof | CHEN JINDING | 6-
Dec- 04 |
6-
Dec -04 |
8-
Jun- 06 |
| US20060122
368 |
Antigen delivery platform | GOVERNMENT OF THE U S A AS REP | 3-
Dec- 04 |
2-
Dec -05 |
8-
Jun- 06 |
| US20060121
580 |
Binding molecules against SARS-coronavirus and uses thereof | CRUCELL | 22-
Jul-03 |
20-
Jan -06 |
8-
Jun- 06 |
| US20060121
043 |
Use of modulators of EphA2 and EphrinAl for the treatment and prevention of infections | MEDIMMUNE, INC. | 27-
Oct- 04 |
27-
Oct -05 |
8-
Jun- 06 |
| US20060115
875 |
Detection, characterization and treatment of viral infection and methods thereof | BROWN EARL | 30-
Sep- 03 |
11-
Oct -05 |
1-
Jun- 06 |
| US20060113
298 |
Electromagnetic wave applicator | ZAIDAN HOJIN HANDOTAI KENKYU SHINKOKAI | 27-
Nov- 02 |
27-
Nov -03 |
1-
Jun- 06 |
| US20060111
387 |
ARYL SUBSTITUTED I MIDAZOQUINOLINES | 3M INNOVATIVE PROPERTIES CO | 20-
Dec- 02 |
13-
Jan -06 |
25-
May -06 |
| US20060110
803 |
Antigenic peptides of SARS coronavirus and uses thereof | Crucell Holland B.V. | 13-
Jun- 03 |
6-
Dec -05 |
25-
May -06 |
| US20060110
758 |
Synthetic peptide targeting critical sites on the SARS-associated coronavirus spike protein responsible for viral infection and method of use thereof | UNIV HONG KONG | 22-
Nov- 04 |
28-
Oct -05 |
25-
May -06 |
| US20060110
405 |
Plasma or serum fraction for treatment and prevention of viral infections and related conditions | BUCKHEIT ROBERT W JR | 20-
Aug- 04 |
22-
Aug -05 |
25-
May -06 |
| US20060100
232 |
Inhibitors of HIV-1 capsid formation: substituted aryl aminomethyl thiazole ureas and analogues thereof | AGARWAL ATUL | 11-
Nov- 04 |
10-
Nov -05 |
11-
May -06 |
| US20060100
229 |
Pyrazolopyridines and analogs thereof | BONK JASON D | 3-
Oct- 03 |
1-
Apr -05 |
11-
May -06 |
| US20060099
606 |
Assay to detect viral uncoating | NARAYAN SHAKTI | 9-
Dec- 03 |
8-
Dec -04 |
11-
May -06 |
| US20060099
573 |
Diagnostic assays | MIGUEZ MARIA-JOSE | 24-
Mar- 04 |
24-
Mar -05 |
11-
May -06 |
| US20060094
105 |
Mixed cell diagnostic systems for detection of respiratory, herpes and enteric viruses | Diagnostic Hybrids, Inc. | 24-
Apr- 98 |
20-
Sep -05 |
4-
May -06 |
| US20060094
104 |
Animal protein-free media for cultivation of cells | DORNER FRIEDRICH | 29-
Oct- 04 |
29-
Oct -04 |
4-
May -06 |
| US20060093
616 |
Process for vaccinating eucaryotic hosts and for protecting against SARS-CoV infection | ALTMEYER RALF | 29-
Sep- 04 |
28-
Sep -05 |
4-
May -06 |
| US20060089
324 |
RNAi modulation of RSV, PIV and other respiratory viruses and uses thereof | BARIK SAILEN | 22-
Oct- 04 |
14-
Jun -05 |
27-
Apr- 06 |
| US20060089
323 |
RNAi modulation of RSV, PIV and other respiratory viruses and uses thereof | BARIK SAILEN | 22-
Oct- 04 |
14-
Jun -05 |
27-
Apr- 06 |
| US20060088
926 |
Method of removing hazardous substance, and hazardous substance removing material using the same such as air cleaning filter, mask and wipping sheet, and method of storing the same | DAIKIN IND LTD | 28-
Mar- 03 |
26-
Mar -04 |
27-
Apr- 06 |
| US20060088
909 |
Virus-like particles, methods of preparation, and immunogenic compositions | COMPANS RICHARD W | 17-
May- 02 |
19-
May -03 |
27-
Apr- 06 |
| US20060079
485 |
Use of lipid conjugates in the treatment of infection | OJCIUS DAVID | 10-
Jan- 00 |
8-
Sep -05 |
13-
Apr- 06 |
| US20060075
894 |
Bioagent air filtration systems | University of Wyoming Research Corporation d/b/a Western Research Institute | 28-
Jan- 03 |
28-
Jan -04 |
13-
Apr- 06 |
| US20060070
952 |
Charge-based water filtration systems | University of Wyoming Research Corporation d/b/a | 28-
Jan- 03 |
28-
Jan -04 |
6-
Apr- 06 |
| US20060067
940 |
Antibodies against West Nile Virus and therapeutic and prophylactic uses thereof | DIAMOND MICHAEL | 21-
Jun- 04 |
21-
Jun -05 |
30-
Mar -06 |
| US20060063
150 |
Antisense antiviral compound and method for treating ssRNA viral infection | IVERSEN PATRICK L | 16-
Sep- 04 |
14-
Sep -05 |
23-
Mar -06 |
| US20060063
149 |
Compositions and methods for detecting pathogen infection | BERTHET FRANCOIS X | 23-
Dec- 03 |
6-
Sep -05 |
23-
Mar -06 |
| US20060062
804 |
SARS-CoV-specific B-cell epitope and applications thereof | National Taiwan University | 21-
Sep- 04 |
21-
Sep -04 |
23-
Mar -06 |
| US20060057
605 |
Compositions for use in identification of viral hemorrhagic fever viruses | ISIS Pharmaceuticals, Inc. | 22-
Mar- 04 |
21-
Mar -05 |
16-
Mar -06 |
| US20060057
161 |
Detection of coronavirus infection | CHOU CHIH-MING | 16-
Sep- 04 |
16-
Sep -04 |
16-
Mar -06 |
| US20060053
516 |
Genetically modified plants comprising SARS-CoV viral nucleotide sequences and methods of use thereof for immunization against SARS | The University of Hong Kong | 5-
Dec- 03 |
3-
Dec -04 |
9-
Mar -06 |
| US20060051
744 |
Feline infectious peritonitis (FIP) and systemic multi-organ coronavirus biomarkers and screening methods | AUSTIN KIMBERLY M | 30-
Jun- 04 |
28-
Jun -05 |
9-
Mar -06 |
| US20060051
374 |
Compositions and methods for mucosal vaccination | 3M Innovative Properties Company | 28-
Apr- 04 |
28-
Apr -05 |
9-
Mar -06 |
| US20060035
926 |
Benzothiazolium compounds | LEE SHIOW-JU | 13-
Aug- 04 |
15-
Aug -05 |
16-
Feb -06 |
| US20060035
859 |
Treating severe and acute viral infections | HEMISPHERx BIOPHARMA | 16-
May- 03 |
6-
Oct -05 |
16-
Feb -06 |
| US20060035
853 |
Methods for tailoring the immune response to an antigen or immunogen | Biomedical Research Models, Inc. | 7-
Jan- 04 |
7-
Jan -05 |
16-
Feb -06 |
| US20060035
327 |
Recombinant super-compound interferon and uses thereof | WEI GUANGWEN | 26-
Aug- 04 |
10-
Mar -05 |
16-
Feb -06 |
| US20060034
853 |
Novel human virus causing respiratory tract infection and uses thereof | CHAN KWOK H | 21-
Jul-04 |
16-
May -05 |
16-
Feb -06 |
| US20060024
668 |
Coronavirus, nucleic acid, protein, and methods for the generation of vaccine, medicaments and diagnostics | HOEK CORNELIA V D | 7-
Jan- 04 |
18-
Aug -04 |
2-
Feb -06 |
| US20060024
271 |
Treatments for viral infections using IFN cytokines and ribavirin, alone or in combination | ALIBEK KEN | 15-
Jan- 04 |
13-
Jan -05 |
2-
Feb -06 |
| US20060019
976 |
Methods for treating Hepatitis C | CHEN GUANGMING | 22-
Jul-04 |
14-
Jul- 05 |
26-
Jan- 06 |
| US20060019
967 |
SARS CoV main protease inhibitors | HSIEH HSING-PANG | 21-
Jul-04 |
20-
Jul- 05 |
26-
Jan- 06 |
| US20060019
927 |
Anti-viral uses of borinic acid complexes | BELLINGER-KAWAHARA CAROLYN | 14-
Jun- 04 |
14-
Jun -05 |
26-
Jan- 06 |
| US20060019
923 |
Methods and compositions for inducing innate immune responses | Coley Pharmaceutical Group, Ltd. | 18-
Jul-04 |
18-
Jul- 05 |
26-
Jan- 06 |
| US20060018
923 |
Novel human virus causing respiratory tract infection and uses thereof | CHAN KWOK H | 21-
Jul-04 |
21-
Jul- 04 |
26-
Jan- 06 |
| US20060018
877 |
Intradermal delivery of vacccines and therapeutic agents | ALARCON JASON B | 29-
Jun- 01 |
29-
Apr -05 |
26-
Jan- 06 |
| US20060014
254 |
Albumin fusion proteins | Human Genome Sciences, Inc. | 22-
Jan- 03 |
7-
Jul- 05 |
19-
Jan- 06 |
| US20060009
459 |
Pyridazine derivatives and their use as therapeutic agents | ABREO MELWYN | 16-
Mar- 04 |
9-
Feb -05 |
12-
Jan- 06 |
| US20060008
810 |
Methods and compositions for detecting rhinoviruses | Focus Technologies, Inc. | 6-Jul-
04 |
6-
Jul- 04 |
12-
Jan- 06 |
| US20060008
379 |
Room decontamination with hydrogen peroxide vapor | STERIS INC. | 8-Jul-
04 |
8-
Jul- 04 |
12-
Jan- 06 |
| US20060006
678 |
Door handle cover | HERRON ROY H JR | 27-
May- 04 |
26-
May -05 |
12-
Jan- 06 |
| US20060003
941 |
Peptides and peptidomimetics having immune-modulating, antiinflammatory, and anti-viral activity | KAWABE TAKUMI | 25-
Jun- 03 |
25-
Jun -04 |
5-
Jan- 06 |
| US20060003
352 |
Mass tag PCR for mutliplex diagnostics | BRIESE THOMAS | 29-
Apr- 04 |
28-
Apr -05 |
5-
Jan- 06 |
| US20060003
351 |
Methods and kits for identifying target nucleotides in mixed populations | Applera Corporation | 30-
Apr- 04 |
29-
Apr -05 |
5-
Jan- 06 |
| US20060003
340 |
Multi-allelic molecular detection of SARS-associated coronavirus | Birch Biomedical Research, LLC | 22-
Aug- 03 |
13-
Aug -04 |
5-
Jan- 06 |
| US20060002
947 |
Ii-key/antigenic epitope hybrid peptide vaccines | HUMPHREYS ROBERT | 14-
Sep- 99 |
11-
Jan -05 |
5-
Jan- 06 |
| US20060002
932 |
Methods and compositions for enhancement of immunity by in vivo depletion of immunosuppressive cell activity | Duke University | 4-
Jun- 04 |
3-
Jun -05 |
5-
Jan- 06 |
| US20050288
866 |
Computational method for identifying adhesin and adhesin-like proteins of therapeutic potential | COUNCIL OF SCIENTIFIC AND INDUSTRIAL RESEARCH | 20-
Jul-04 |
7-
Feb -05 |
29-
Dec -05 |
| US20050287
167 |
Polycistronic HIV vector constructs | 5-
May- 04 |
5-
May -05 |
29-
Dec -05 |
|
| US20050287
118 |
Bacterial plasmid with immunological adjuvant function and uses thereof | 26-
Nov- 03 |
23-
Nov -04 |
29-
Dec -05 |
|
| US20050282
279 |
Expression vector encoding coronavirus-like particle | HWU PAUL L | 29-
May- 03 |
28-
May -04 |
22-
Dec -05 |
| US20050282
209 |
Variable length probe selection | ALBERT THOMAS | 18-
Jun- 04 |
20-
Jun -05 |
22-
Dec -05 |
| US20050282
154 |
Angiotensin-converting enzyme-2 as a receptor for the SARS coronavirus | 6-
Oct- 03 |
5-
Oct -04 |
22-
Dec -05 |
|
| US20050281
828 |
Method of treating autoimmune disease by inducing antigen presentation by tolerance inducing antigen presenting cells | BOWDISH KATHERINE S | 28-
Feb- 04 |
1-
Apr -05 |
22-
Dec -05 |
| US20050277
592 |
Beta-peptides | Yale University | 21-
Apr- 04 |
21-
Apr -05 |
15-
Dec -05 |
| US20050277
181 |
Compositions and methods for detecting pathogen infection | BERTHET FRANCOIS X | 23-
Dec- 04 |
27-
Apr -05 |
15-
Dec -05 |
| US20050276
818 |
Uncharacterized ORF3 in SARS-coronavirus is a cyclic-AMP- dependent kinase and a target for SARS therapy | GODZIK ADAM | 17-
May- 04 |
17-
May -05 |
15-
Dec -05 |
| US20050276
815 |
Antiviral activity from medicinal mushrooms | STAMETS PAUL | 6-
Jan- 04 |
6-
Jan -05 |
15-
Dec -05 |
| US20050271
711 |
Therapeutic antimicrobial compositions and methods | The Procter & Gamble Company | 26-
Apr- 04 |
25-
Apr -05 |
8-
Dec -05 |
| US20050266
550 |
TC-83-derived alphavirus vectors, particles and methods | Alphavax, Inc. | 18-
May- 04 |
18-
May -05 |
1-
Dec -05 |
| US20050266
465 |
Interferon-alpha polypeptides and conjugates | 19-
May- 04 |
18-
May -05 |
1-
Dec -05 |
|
| US20050266
425 |
Methods for producing and identifying multispecific antibodies | Vaccinex, Inc. | 31-
Dec- 03 |
29-
Dec -04 |
1-
Dec -05 |
| US20050266
397 |
Methods for identification of coronaviruses | BLYN LAWRENCE B | 26-
Apr- 03 |
22-
Apr -04 |
1-
Dec -05 |
| US20050260
645 |
Comparative genomic resequencing | ALBERT THOMAS | 8-
Apr- 04 |
8-
Apr -05 |
24-
Nov -05 |
| US20050255
123 |
Chimeric ebola virus envelopes and uses therefor | The Trustees of the University of Pennsylvania | 30-
Apr- 02 |
28-
Apr -03 |
17-
Nov -05 |
| US20050250
677 |
Glycopeptide antibiotic derivatives | BALZARINI JAN | 1-
Sep- 03 |
1-
Sep -03 |
10-
Nov -05 |
| US20050249
742 |
Compositions and methods for modulating a cytotoxic T lymphocyte immune response | Dana-Farber Cancer Institute, Inc. | 27-
Jun- 02 |
22-
Dec -04 |
10-
Nov -05 |
| US20050249
739 |
Antibodies against SARS-CoV and methods of use thereof | MARASCO WAYNE | 25-
Nov- 03 |
24-
Nov -04 |
10-
Nov -05 |
| US20050244
423 |
Methods for treating viral infection using IL-28 and IL-29 cysteine mutants | HENDERSON KATHERINE E | 2-
Apr- 04 |
4-
Apr -05 |
3-
Nov -05 |
| US20050240
353 |
Reagents, devices and methods for proteomic analysis with applications including diagnostics, vaccines, quality control and research | 21-
Apr- 04 |
4-
Feb -05 |
27-
Oct- 05 |
|
| US20050239
735 |
Enhancement of immune responses | 30-
Dec- 03 |
30-
Dec -04 |
27-
Oct- 05 |
|
| US20050239
086 |
Multiplex systems, methods, and kits for detecting and identifying nucleic acids | BRIESE THOMAS | 27-
Apr- 04 |
27-
Apr -04 |
27-
Oct- 05 |
| US20050239
047 |
Methods and devices for determining a cell characteristic, and applications employing the same | GIMZEWSKI JAMES K | 10-
Sep- 02 |
9-
Mar -05 |
27-
Oct- 05 |
| US20050238
655 |
Antiviral activity from medicinal mushrooms | STAMETS PAUL | 6-
Jan- 04 |
4-
Jan -05 |
27-
Oct- 05 |
| US20050234
122 |
Prevention of and countermeasures against viral infection | CHOKO CO., LTD. | 11-
Mar- 04 |
11-
Mar -04 |
20-
Oct- 05 |
| US20050233
314 |
Sensitive and quantitative detection of pathogens by real-time nested PCR | 30-
Jun- 03 |
30-
Jun -04 |
20-
Oct- 05 |
|
| US20050232
895 |
Anti-viral pharmaceutical compositions | SD Pharmaceuticals, Inc. | 10-
Dec- 03 |
3-
Dec -04 |
20-
Oct- 05 |
| US20050223
427 |
Modified polynucleotides for reducing off-target effects in RNA interference | Dharmacon, Inc. | 1-
Apr- 04 |
22-
Dec -04 |
6-
Oct- 05 |
| US20050222
258 |
Pharmaceuticals comprising shikonins as active constituent | WANG FEIXIN | 21-
Feb- 03 |
14-
May -05 |
6-
Oct- 05 |
| US20050220
816 |
Mutant viral nucleic acids and vaccine containing same | 31-
Mar- 04 |
31-
Mar -04 |
6-
Oct- 05 |
|
| US20050215
494 |
Baicalin and its derivatives as a treatment for SARS coronavirus infection or other related infections | CHAN KWOK H | 10-
Nov- 03 |
8-
Nov -04 |
29-
Sep -05 |
| US20050214
890 |
Novel „Cleave-N-Read” system for protease activity assay and methods of use thereof | BAUDRY MICHEL | 26-
Nov- 03 |
23-
Nov -04 |
29-
Sep -05 |
| US20050214
748 |
Peptide-based diagnostic reagents for SARS | CHANG TSENG Y | 12-
Nov- 03 |
8-
Nov -04 |
29-
Sep -05 |
| US20050214
747 |
Compositions and methods for analysis of target analytes | DANIELZADEH ROBERT | 16-
Jan- 04 |
17-
Oct -04 |
29-
Sep -05 |
| US20050208
066 |
Recombinant baculovirus and virus-like particle | CHAO YU-CHAN | 25-
Nov- 03 |
24-
Nov -04 |
22-
Sep -05 |
| US20050208
060 |
Vaccine composition | Aventis Pasteur S.A. | 22-
Sep- 04 |
15-
Nov -04 |
22-
Sep -05 |
| US20050208
020 |
Enhancement of vaccine-induced immune responses and protection by heterologous boosting with alphavirus replicon vaccines | BRICE GARY T | 12-
Nov- 03 |
10-
Nov -04 |
22-
Sep -05 |
| US20050208
019 |
Uses of interferons with altered spatial structure | WEI GUANGWEN | 28-
Aug- 03 |
26-
Aug -04 |
22-
Sep -05 |
| US20050203
038 |
Modulation of ACE2 expression | 10-
Mar- 04 |
10-
Mar -04 |
15-
Sep -05 |
|
| US20050203
024 |
Peptides and methods for inducing cellular resistance to infection | 1-
Dec- 03 |
1-
Dec -04 |
15-
Sep -05 |
|
| US20050202
415 |
Replikin peptides and uses thereof | BOGOCH ELENORE S. | 23-
Dec- 03 |
4-
Jun -04 |
15-
Sep -05 |
| US20050196
382 |
Antiviral oligonucleotides targeting viral families | 5-
Dec- 02 |
19-
Oct -04 |
8-
Sep -05 |
|
| US20050196
381 |
Lentivirus vector-based approaches for generating an immune response to HIV in humans | DROPULIC BORO | 9-
Sep- 03 |
9-
Sep -04 |
8-
Sep -05 |
| US20050191
620 |
Particle on membrane assay system | BALLARD KARRI L. | 27-
Feb- 04 |
22-
Dec -04 |
1-
Sep -05 |
| US20050189
302 |
Viral inactivation using ozone | KEYSER STEVEN A. | 17-
Mar- 04 |
11-
Oct -04 |
1-
Sep -05 |
| US20050187
192 |
Phospholipids for the treatment of infection by togaviruses, herpes viruses and coronaviruses | 20-
Feb- 04 |
20-
Feb -04 |
25-
Aug -05 |
|
| US20050186
575 |
Corona-virus-like particles comprising functionally deleted genomes | BOSCH BEREND J. | 17-
May- 02 |
30-
Dec -03 |
25-
Aug -05 |
| US20050182
243 |
Membrane scaffold proteins | BAYBURT TIMOTHY H. | 18-
Jun- 03 |
11-
Jan -05 |
18-
Aug -05 |
| US20050181
357 |
High-throughput diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS) | CHAN KWOK H. | 24-
Mar- 03 |
24-
Mar -04 |
18-
Aug -05 |
| US20050181
075 |
Pharmacological enhancement and manufacturing method of antiviral compound | GONG JIAO | 21-
Nov- 03 |
21-
Nov -03 |
18-
Aug -05 |
| US20050176
661 |
Antiviral oligonucleotides | 5-
Dec- 02 |
12-
Sep -03 |
11-
Aug -05 |
|
| US20050171
044 |
Oligonucleotide compound and method for treating nidovirus infections | BESTWICK RICHARD K. | 24-
Dec- 03 |
22-
Dec -04 |
4-
Aug -05 |
| US20050169
885 |
Recombinant super-compound interferon | WEI GUANGWEN | 28-
Aug- 03 |
26-
Aug -04 |
4-
Aug -05 |
| US20050164
167 |
Method of discovery and development of broad-spectrum antiviral drugs | BUSCHER BENJAMIN A. | 28-
Jan- 04 |
28-
Jan -04 |
28-
Jul- 05 |
| US20050163
648 |
METHOD AND APPARATUS FOR STERILIZING AIR IN LARGE VOLUMES BY RADIATION OF ULTRAVIOLET RAYS | LIANG MICHAEL Y. | 26-
Jan- 04 |
26-
Jan -04 |
28-
Jul- 05 |
| US20050158
411 |
Methods and apparatus to prevent, treat, and cure the symptoms of nauea caused by chemotherapy treatments of human cancers | VAIL MARILYN L. | 8-
Dec- 03 |
27-
Nov -04 |
21-
Jul- 05 |
| US20050158
325 |
Immunomodulatory combinations | 30-
Dec- 03 |
30-
Dec -04 |
21-
Jul- 05 |
|
| US20050153
912 |
Antiviral oligonucleotides targeting viral families | 5-
Dec- 02 |
12-
Sep -03 |
14-
Jul- 05 |
|
| US20050153
310 |
Luciferase biosensor | BUTLER BRAEDEN | 10-
Oct- 03 |
1-
Oct -04 |
14-
Jul- 05 |
| US20050148
658 |
Method for preventing and treating severe acute respiratory syndrome | HENSLEY CHARLES | 5-
Jan- 04 |
5-
Jan -04 |
7-
Jul- 05 |
| US20050147
697 |
Compositions and methods for reducing the transmissivity of illnesses | ROSENBLOOM RICHARD A. | 6-
Feb- 03 |
14-
Dec -04 |
7-
Jul- 05 |
| US20050142
536 |
Method and kit for the detection of a novel coronoavirus associated with the severe acute respiratory syndrome (SARS) | LAUE THOMAS | 30-
Apr- 03 |
30-
Apr -04 |
30-
Jun- 05 |
| US20050142
157 |
Oxidative reductive potential water solution and methods of using the same | Oculus Innovative Sciences, Inc. | 30-
Dec- 03 |
11-
Aug -04 |
30-
Jun- 05 |
| US20050139
808 |
Oxidative reductive potential water solution and process for producing same | Oculus Innovative Sciences, Inc. | 30-
Dec- 03 |
4-
Jun -04 |
30-
Jun- 05 |
| US20050123
563 |
Lipoparticles comprising proteins, methods of making, and using the same | DORANZ BENJAMIN J. | 30-
Jul-03 |
28-
Jul- 04 |
9-
Jun- 05 |
| US20050119
284 |
ANTIVIRAL AGENTS AND METHODS OF USE | Wisconsin Alumni Research Foundation – | 30-
Sep- 03 |
30-
Sep -04 |
2-
Jun- 05 |
| US20050119
251 |
Nicotinamide derivatives and their use as therapeutic agents | FINE RICHARD M. | 21-
Dec- 01 |
6-
Jul- 04 |
2-
Jun- 05 |
| US20050117
187 |
Apparatus for forming nano-grating device | CHEN YUNG-HSIN | 17-
Mar- 04 |
17-
Mar -04 |
2-
Jun- 05 |
| US20050114
910 |
Transgenic mice having a human major histocompatibility complex (MHC) phenotype, experimental uses and applications | AURIAULT CLAUDE | 30-
Jul-03 |
2-
Jul- 04 |
26-
May -05 |
| US20050113
298 |
Receptor binding peptides derived from the SARS S protein | 15-
Sep- 03 |
13-
Sep -04 |
26-
May -05 |
|
| US20050112
559 |
Compositions and methods for diagnosing and preventing severe acute respiratory syndrome (SARS) | 29-
Sep- 03 |
29-
Sep -04 |
26-
May -05 |
|
| US20050112
558 |
Prognostic PCR assay for severe acute respiratory syndrome (SARS) | The Chinese University of Hong Kong | 24-
Sep- 03 |
23-
Sep -04 |
26-
May -05 |
| US20050112
555 |
Cytidine deaminase activators, deoxycytidine deaminase activators, Vif antagonists, and methods of screening for molecules thereof | DEWHURST STEPHEN | 3-
Sep- 03 |
3-
Sep -04 |
26-
May -05 |
| US20050112
554 |
Characterization of the earliest stages of the severe acute respiratory syndrome (SARS) virus and uses thereof | HENG XU RUI | 9-Jul-
04 |
9-
Jul- 04 |
26-
May -05 |
| US20050107
324 |
Modulation of CEACAM1 expression | BENNETT C. F. | 12-
Jul-03 |
12-
Jul- 04 |
19-
May -05 |
| US20050106
563 |
Epitope profiles of SARS coronavirus | 8-
Sep- 03 |
8-
Sep -04 |
19-
May -05 |
|
| US20050101
581 |
Therapeutic treatment methods 2 | AHLEM CLARENCE N. | 28-
Aug- 02 |
5-
Dec -03 |
12-
May -05 |
| US20050100
885 |
Compositions and methods for the treatment of severe acute respiratory syndrome (SARS) | BAKER BRENDA F. | 28-
Apr- 03 |
26-
Apr -04 |
12-
May -05 |
| US20050100
883 |
Peptide-based diagnostic reagents for SARS | CHANG TSENG Y. | 12-
Nov- 03 |
12-
Nov -03 |
12-
May -05 |
| US20050100
612 |
Virucidal activities of cetylpyridinium chloride | ViraTox, L.L.C. | 7-
Nov- 03 |
10-
Sep -04 |
12-
May -05 |
| US20050096
259 |
Neutrophil activation by immune response modifier compounds | 31-
Oct- 03 |
1-
Nov -04 |
5-
May -05 |
|
| US20050095
618 |
Compositions and methods for diagnosing and treating severe acute respiratory syndrome (SARS) | The Chinese University of Hong Kong | 29-
Jul-03 |
28-
Jul- 04 |
5-
May -05 |
| US20050095
582 |
Compositions and methods for detecting severe acute respiratory syndrome coronavirus | Diagnostic Hybrids, Inc. | 3-
Nov- 03 |
3-
Nov -03 |
5-
May -05 |
| US20050090
505 |
Methods of reducing risk of infection from pathogens | HOPKINS SAMUEL E. | 20-
Aug- 03 |
18-
Aug -04 |
28-
Apr- 05 |
| US20050085
529 |
Prénylation inhibitors reduce host cell permissiveness to viral replication | 21-
Oct- 03 |
21-
Oct -03 |
21-
Apr- 05 |
|
| US20050080
093 |
Methods of reducing risk of infection from pathogens | HOPKINS SAMUEL E. | 18-
Aug- 04 |
18-
Aug -04 |
14-
Apr- 05 |
| US20050075
307 |
Modulation of aminopeptidase N expression | BENNETT C. FRANK | 12-
Jul-03 |
12-
Jul- 04 |
7-
Apr- 05 |
| US20050074
743 |
Method and composition for treating a biological sample | CHAPMAN JOHN | 6-
Oct- 03 |
6-
Oct -03 |
7-
Apr- 05 |
| US20050074
359 |
Aircraft and passenger decontamination system | STERIS INC. | 6-
Oct- 03 |
6-
Oct -03 |
7-
Apr- 05 |
| US20050071
892 |
Techniques and applications of establishment of SARS-CoV primate model | GAO HONG | 27-
Jun- 03 |
25-
Jun -04 |
31-
Mar -05 |
| US20050070
460 |
Infection prophylaxis using immune response modifier compounds | 3M Innovative Properties Company | 5-
Aug- 03 |
5-
Aug -04 |
31-
Mar -05 |
| US20050069
911 |
Proteome epitope tags and methods of use thereof in protein modification analysis | engeneOS, Inc. | 10-
May- 02 |
5-
Feb -04 |
31-
Mar -05 |
| US20050069
869 |
SARS nucleic acids, proteins, antibodies, and uses thereof | AMBROSINO DONNA | 4-
Aug- 03 |
4-
Aug -04 |
31-
Mar -05 |
| US20050069
558 |
Crystals and structures of SARS-CoV main protease | BONANNO JEFFREY B. | 25-
Jul-03 |
23-
Jul- 04 |
31-
Mar -05 |
| US20050069
555 |
Thiosemicarbazones as anti-virals and immunopotentiators | BARSANTI PAUL A. | 27-
Dec- 02 |
29-
Dec -03 |
31-
Mar -05 |
| US20050065
143 |
Pyridazine derivatives and their use as therapeutic agents | Xenon Pharmaceuticals Inc. | 29-
Jul-04 |
29-
Jul- 04 |
24-
Mar -05 |
| US20050059
578 |
Compositions comprising phosphatidylethanolamine-binding peptides linked to anti-viral agents | HE JIN | 15-
Aug- 03 |
15-
Aug -03 |
17-
Mar -05 |
| US20050059
576 |
Targeted delivery of antiviral compounds through hemoglobin bioconjugates | ADAMSON J. GORDON | 30-
Apr- 98 |
17-
May -04 |
17-
Mar -05 |
| US20050059
072 |
Selective modulation of TLR gene expression | 3M Innovative Properties Company | 17-
Sep- 04 |
17-
Sep -04 |
17-
Mar -05 |
| US20050058
982 |
Modified small interfering RNA molecules and methods of use | Chiron Corporation | 25-
Jul-03 |
25-
Jul- 03 |
17-
Mar -05 |
| US20050054
644 |
Hydrolytically-resistant boron-containing therapeutics and methods of use | AKAMA TSUTOMU | 15-
Jun- 04 |
15-
Jun -04 |
10-
Mar -05 |
| US20050054
640 |
1-Amino lH-imidazoquinolines | GRIESGRABER GEORGE W. | 3-
Sep- 04 |
3-
Sep -04 |
10-
Mar -05 |
| US20050053
990 |
Cleavage of RNA by restriction endonucleases | ROBERTS RICHARD J. | 31-
Aug- 04 |
31-
Aug -04 |
10-
Mar -05 |
| US20050051
497 |
Viral inactivation using ozone | KEYSER STEVEN A. | 2-
Aug- 04 |
2-
Aug -04 |
10-
Mar -05 |
| US20050048
473 |
Enzymatic diagnostic test for SARS and other viral diseases | ARAD DORIT | 23-
Jun- 04 |
23-
Jun -04 |
3-
Mar -05 |
| US20050048
465 |
Method of collecting nasopharyngeal cells and secretions for diagnosis of viral upper respiratory infections and screening for nasopharyngeal cancer | World Sense Technology Limited | 20-
Aug- 04 |
20-
Aug -04 |
3-
Mar -05 |
| US20050048
072 |
Immunostimulatory combinations and treatments | 3M Innovative Properties Company | 25-
Aug- 04 |
25-
Aug -04 |
3-
Mar -05 |
| US20050039
220 |
Imageable animal model of SARS infection | XU MINGXU | 27-
May- 04 |
27-
May -04 |
17-
Feb -05 |
| US20050037
338 |
Method and means for detection of severe acute respiratory syndrome | TSENG KUO-TANG | 14-
Aug- 03 |
14-
Aug -03 |
17-
Feb -05 |
| US20050036
951 |
Methods of treating lung diseases | Arizeke Pharmaceuticals, Inc. | 9-
Jan- 04 |
9-
Jan -04 |
17-
Feb -05 |
| US20050032
222 |
Modified viral particles with immunogenic properties and reduced lipid content useful for treating and preventing infectious diseases | BELLOTTI MARC | 29-
Jun- 00 |
21-
Jun -04 |
10-
Feb -05 |
| US20050031
620 |
Combined cancer treatment methods using selected antibodies to aminophospholipids | HUANG XIANMING | 15-
Aug- 03 |
15-
Aug -03 |
10-
Feb -05 |
| US20050031
592 |
Methods and compositions for inducing immune responses and protective immunity by priming with alpha virus replicon vaccines | BRICE GARY L. | 13-
Nov- 03 |
13-
Nov -03 |
10-
Feb -05 |
| US20050025
788 |
Systemic delivery of non-viral vector expressing SARS viral genomic vaccine | CHOU GEORGE CHIN-SHENG | 4-
Jun- 04 |
4-
Jun -04 |
3-
Feb -05 |
| US20050025
761 |
Anti-viral treatment methods using phosphatidylethanolaminebinding peptides linked to anti-viral agents | Board of Regents, The University of Texas System | 15-
Aug- 03 |
15-
Aug -03 |
3-
Feb -05 |
| US20050020
524 |
Hematopoietic stem cell gene therapy | Monash University | 15-
Apr- 99 |
30-
Dec -03 |
27-
Jan- 05 |
| US20050019
756 |
Methods and compositions for use in diagnosing and characterizing diseases involving abnormal apoptosis | DE MEIRLEIR KENNY | 26-
May- 04 |
26-
May -04 |
27-
Jan- 05 |
| US20050015
847 |
Compositions and methods for preventing infection | HILDRETH JAMES E. | 8-
Aug- 03 |
8-
Aug -03 |
27-
Jan- 05 |
| US20050014
830 |
Substances for breaking down conformation of microbes | CHU SHU FANG | 17-
Jul-03 |
4-
Jun -04 |
20-
Jan- 05 |
| US20050014
752 |
Prodrugs of heteroaryl compounds | Koronis Pharmaceuticals, Incorporated | 31-
Mar- 04 |
31-
Mar -04 |
20-
Jan- 05 |
| US20050009
110 |
Methods of producing antibodies for diagnostics and therapeutics | CHANG XIAO-JIA | 8-Jul-
03 |
8-
Jul- 03 |
13-
Jan- 05 |
| US20050009
009 |
Diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS) | CHAN KWOK HUNG | 24-
Mar- 04 |
24-
Mar -04 |
13-
Jan- 05 |
| US20050008
535 |
Noble gas-chlorine mixture effective against micro organisms | GLOBUS ALFRED R. | 7-Jul-
03 |
7-
Jul- 03 |
13-
Jan- 05 |
| US20050004
144 |
Combined use of IMPDH inhibitors with toll-like receptor agonists | REGENTS OF THE UNIVERSITY OF CALIFORNIA | 14-
Apr- 04 |
14-
Apr -04 |
6-
Jan- 05 |
| US20050004
071 |
Charged polysaccharides resistant to lysosomal degradation during kidney filtration and renal passage and their use to treat or prevent infection by coronaviruses | COMPER WAYNE D. | 20-
Apr- 04 |
20-
Apr -04 |
6-
Jan- 05 |
| US20050004
063 |
Inhibition of SARS-associated coronavirus (SCoV) infection and replication by RNA interference | GUAN YI | 19-
May- 04 |
19-
May -04 |
6-
Jan- 05 |
| US20050003
340 |
Assay system and methods for detecting SARS-CV | AsiaGEN Corporation | 1-Jul-
03 |
1-
Jul- 03 |
6-
Jan- 05 |
| US20050002
953 |
SARS-coronavirus virus-like particles and methods of use | HEROLD JENS | 4-
May- 04 |
4-
May -04 |
6-
Jan- 05 |
| US20050002
941 |
Combinations and kits for cancer treatment using selected antibodies to aminophospholipids | Board of Regents, The University of Texas System | 15-
Aug- 03 |
15-
Aug -03 |
6-
Jan- 05 |
| US20050002
913 |
Hematopoietic stem cell gene therapy | Monash University | 15-
Apr- 99 |
18-
Apr -03 |
6-
Jan- 05 |
| US20050002
901 |
Compositions and methods for treating coronavirus infection and SARS | BLATT LAWRENCE M. | 30-
Mar- 04 |
30-
Mar -04 |
6-
Jan- 05 |
| US20040265
879 |
Compositions for enhancing transport of molecules into cells | IVERSEN PATRICK L. | 29-
Apr- 04 |
29-
Apr -04 |
30-
Dec -04 |
| US20040265
796 |
Methods and kits for detecting SARS-associated coronavirus | BRIESE THOMAS | 23-
Jan- 04 |
23-
Jan -04 |
30-
Dec -04 |
| US20040265
367 |
Liposomes coated with selected antibodies that bind to aminophospholipids | HUANG XIANMING | 15-
Aug- 03 |
15-
Aug -03 |
30-
Dec -04 |
| US20040265
351 |
Methods and compositions for enhancing immune response | KEDL ROSS M. | 9-
Apr- 04 |
9-
Apr -04 |
30-
Dec -04 |
| US20040259
934 |
Inhibiting Coronaviridae viral replication and treating Coronaviridae viral infection with nucleoside compounds | CARROLL STEVEN S. | 27-
Apr- 04 |
27-
Apr -04 |
23-
Dec -04 |
| US20040259
803 |
Disease prevention by reactivation of the thymus | Monash University | 15-
Apr- 99 |
30-
Dec -03 |
23-
Dec -04 |
| US20040258
698 |
Delivery of immune response modifier compounds | JING NAIYONG | 9-
Apr- 04 |
9-
Apr -04 |
23-
Dec -04 |
| US20040253
624 |
Microporous materials, methods of making, using, and articles thereof | DURTSCHI JACOB | 26-
May- 04 |
26-
May -04 |
16-
Dec -04 |
| US20040253
328 |
Anti-atypical pneumonia decoction | HU XIN YUAN | 13-
Jun- 03 |
21-
Jul- 03 |
16-
Dec -04 |
| US20040241
842 |
Stimulation of thymus for vaccination development | Monash University | 15-
Apr- 99 |
30-
Dec -03 |
2-
Dec -04 |
| US20040237
198 |
Protecting shield for performing the insertion of a tube during emergency rescuing or anesthesia | YANG JUI KUANG | 2-
Jun- 03 |
2-
Jun -03 |
2-
Dec -04 |
| US20040235
952 |
Inhibitors of severe acute respiratory syndrome (SARS) 3 C-like proteinase | Agouron Pharmaceuticals, Inc. | 27-
Apr- 04 |
27-
Apr -04 |
25-
Nov -04 |
| US20040235
946 |
Organosulphur prodrugs for the prevention and treatment of injectious diseases and pathologenic immune system response | OTT DAVID M. | 24-
May- 04 |
24-
May -04 |
25-
Nov -04 |
| US20040235
047 |
Compositions and methods for treatment of Severe Acute Respiratory Syndrome (SARS) | SIBER GEORGE R. | 20-
May- 03 |
20-
May -03 |
25-
Nov -04 |
| US20040234
457 |
Methods of preventing and treating SARS using low pH respiratory tract compositions | The Procter & Gamble Company | 2-
Feb- 04 |
2-
Feb -04 |
25-
Nov -04 |
| US20040229
828 |
Antiviral oligonucleotides targeting RSV | Replicor, Inc. | 12-
Sep- 03 |
12-
Sep -03 |
18-
Nov -04 |
| US20040229
778 |
Pharmaceutical compositions of antithrombin III for the treatment of retroviral diseases | ELMALEH DAVID R. | 13-
May- 03 |
13-
May -03 |
18-
Nov -04 |
| US20040229
219 |
Method of inhibiting human metapneumovirus and human coronavirus in the prevention and treatment of severe acute respiratory syndrome (SARS) | GALLAHER WILLIAM R. | 29-
Apr- 04 |
29-
Apr -04 |
18-
Nov -04 |
| US20040229
211 |
Sensitive diagnostic testing methodology using multiplex real time PCR with one dye (MOD) and its use as in severe acute respiratory syndrome (SARS) | YEUNG WAH HIN ALEX | 22-
Aug- 03 |
22-
Aug -03 |
18-
Nov -04 |
| US20040220
139 |
Inhibiting viral infections | SCEUSA NICHOLAS A. | 5-
Nov- 03 |
5-
Nov -03 |
4-
Nov -04 |
| US20040219
155 |
Selected immunoconjugates for binding to aminophospholipids | RAN SOPHIA | 15-
Aug- 03 |
15-
Aug -03 |
4-
Nov -04 |
| US20040214
785 |
Surface sanitizing compositions with improved antimicrobial performance | Xantech Pharmaceuticals, Inc. | 9-
Mar- 04 |
9-
Mar -04 |
28-
Oct- 04 |
| US20040214
764 |
Anti-viral treatment methods using phosphatidylethanolaminebinding peptide derivatives | HE JIN | 15-
Aug- 03 |
15-
Aug -03 |
28-
Oct- 04 |
| US20040213
779 |
Methods for treating viral infections using immunoconjugates to aminophospholipids | RAN SOPHIA | 15-
Aug- 03 |
15-
Aug -03 |
28-
Oct- 04 |
| US20040209
844 |
Compositions and methods for preventing infection | HILDRETH JAMES E. | 22-
Sep- 03 |
22-
Sep -03 |
21-
Oct- 04 |
| US20040208
873 |
Human monoclonal antibodies against interleukin 8 (IL-8) | GENMAB A/S | 16-
Dec- 03 |
16-
Dec -03 |
21-
Oct- 04 |
| US20040208
868 |
Selected antibody CDRs for binding to aminophospholipids | Board of Regents, The University of Texas System | 15-
Aug- 03 |
15-
Aug -03 |
21-
Oct- 04 |
| US20040204
420 |
Compounds for modulating RNA interference | RANA TARIQ M. | 5-
Aug- 03 |
5-
Aug -03 |
14-
Oct- 04 |
| US20040202
720 |
Delivery of immune response modifier compounds using metal- containing particulate support materials | 3M Innovative Properties Company | 9-
Apr- 04 |
9-
Apr -04 |
14-
Oct- 04 |
| US20040191
833 |
Selective activation of cellular activities mediated through a common toll-like receptor | 3M Innovative Properties Company | 24-
Mar- 04 |
24-
Mar -04 |
30-
Sep -04 |
| US20040184
950 |
Building decontamination with vaporous hydrogen peroxide | STERIS INC. | 29-
Jan- 04 |
29-
Jan -04 |
23-
Sep -04 |
| US20040180
380 |
Proteome epitope tags and methods of use thereof in protein modification analysis | engeneOS, Inc. | 13-
Nov- 03 |
13-
Nov -03 |
16-
Sep -04 |
| US20040176
367 |
1-Amino lH-imidazoquinolines | 3M Innovative Properties Company | 5-
Mar- 04 |
5-
Mar -04 |
9-
Sep -04 |
| US20040175
378 |
Selected antibody compositions and methods for binding to aminophospholipids | Board of Regents, The University of Texas System | 15-
Jul-03 |
15-
Jul- 03 |
9-
Sep -04 |
| US20040171
568 |
Antiviral oligonucleotides targeting HIV | Replicor, Inc. | 12-
Sep- 03 |
12-
Sep -03 |
2-
Sep -04 |
| US20040171
086 |
Selective modulation of TLR-mediated biological activity | 3M Innovative Properties Company | 27-
Feb- 04 |
27-
Feb -04 |
2-
Sep -04 |
| US20040170
965 |
Mixed cell diagnostic systems | GOODRUM PATRICIA GAIL RAY | 30-
Mar- 04 |
30-
Mar -04 |
2-
Sep -04 |
| US20040170
959 |
Methods for identifying antiviral oligonucleotides | Replicor, Inc. | 11-
Sep- 03 |
12-
Sep -03 |
2-
Sep -04 |
| US20040170
649 |
Method of treating and preventing infectious diseases via creation of a modified viral particle with immunogenic properties | CHAM BILL E. | 29-
Jun- 00 |
20-
Jun -03 |
2-
Sep -04 |
| US20040170
620 |
Selected antibody compositions for binding to aminophospholipids | RAN SOPHIA | 15-
Jul-03 |
15-
Jul- 03 |
2-
Sep -04 |
| US20040167
161 |
Method of treating or inhibiting the development of brain inflammation and sepsis | NOZAKI MASAKO | 20-
May- 03 |
26-
Nov -03 |
26-
Aug -04 |
| US20040167
073 |
Casein derived peptides and uses thereof | Chay 13 Medical Research Group N.V. | 1-
Mar- 00 |
1-
Mar -04 |
26-
Aug -04 |
| US20040162
309 |
Methods and compositions related to IRM compounds and toll-like receptor 8 | 3M Innovative Properties Company | 12-
Feb- 04 |
12-
Feb -04 |
19-
Aug -04 |
| US20040162
254 |
Antiviral oligonucleotides targeting HSV and CMV | Replicor, Inc. | 12-
Sep- 03 |
12-
Sep -03 |
19-
Aug -04 |
| US20040162
253 |
Antiviral oligonucleotides targeting HBV | Replicor, Inc. | 12-
Sep- 03 |
12-
Sep -03 |
19-
Aug -04 |
| US20040147
543 |
Aryl substituted imidazoquinolines | 3M Innovative Properties Company | 18-
Dec- 03 |
18-
Dec -03 |
29-
Jul- 04 |
| US20040147
440 |
Compositions comprising cell-impermeant duramycin derivatives | Board of Regents, The University of Texas System | 15-
Aug- 03 |
15-
Aug -03 |
29-
Jul- 04 |
| US20040142
852 |
Composition and its therapeutic use | AL SARI AHMAD M. H. | 9-
May- 02 |
29-
May -03 |
22-
Jul- 04 |
| US20040142
322 |
Continuous non-radioactive polymerase assay | Schering Corporation | 7-
Oct- 03 |
7-
Oct -03 |
22-
Jul- 04 |
| US20040141
950 |
Immunostimulatory combinations | 3M Innovative Properties Company | 30-
Dec- 03 |
30-
Dec -03 |
22-
Jul- 04 |
| US20040138
187 |
Therapeutic treatment methods | AHLEM CLARENCE N. | 28-
Aug- 03 |
28-
Aug -03 |
15-
Jul- 04 |
| US20040131
622 |
Combinations and kits for treating viral infections using immunoconjugates to aminophospholipids | Board of Regents | 15-
Aug- 03 |
15-
Aug -03 |
8-
Jul- 04 |
| US20040131
621 |
Combinations and kits for treating viral infections using antibodies to aminophospholipids | RAN SOPHIA | 15-
Aug- 03 |
15-
Aug -03 |
8-
Jul- 04 |
| US20040131
610 |
Methods for treating viral infections using antibodies to aminophospholipids | RAN SOPHIA | 15-
Aug- 03 |
15-
Aug -03 |
8-
Jul- 04 |
| US20040081
667 |
Compositions and methods for treating and preventing infection | HILDRETH JAMES E. | 22-
Jul-03 |
22-
Jul- 03 |
29-
Apr- 04 |
| US20040077
587 |
2′-C-methyl-3′-O-L-valine ester ribofuranosyl cytidine for treatment of flaviviridae infections | LACOLLA PAOLA | 27-
Jun- 03 |
27-
Jun -03 |
22-
Apr- 04 |
| US20040071
757 |
Inhalation antiviral patch | ROLF DAVID | 10-
Jun- 03 |
10-
Jun -03 |
15-
Apr- 04 |
| US20040071
709 |
Corona-virus-like particles comprising functionally deleted genomes | BOSCH BEREND-JAN | 17-
May- 01 |
14-
Apr -03 |
15-
Apr- 04 |
| US20040067
920 |
Nebulizer formulations of dehydroepiandrosterone and methods of treating asthma or chronic obstructive pulmonary disease using compositions thereof | JOHNSON KEITH A. | 17-
Jun- 03 |
17-
Jun -03 |
8-
Apr- 04 |
| US20040009
943 |
Pathogen vaccines and methods for using the same | Inex Pharmaceuticals Corporation | 12-
May- 03 |
12-
May -03 |
15-
Jan- 04 |
| US20040009
245 |
Methods and apparatus to prevent, treat and cure infections of the human respiratory system by pathogens causing severe acute respiratory syndrome (SARS) | VAIL MARILYN L. | 2-
May- 03 |
2-
May -03 |
15-
Jan- 04 |
| US20040009
152 |
Materials and methods for prevention and treatment of RNA viral diseases | BEHERA ARUNA K. | 30-
Apr- 03 |
30-
Apr -03 |
15-
Jan- 04 |
| US20040005
998 |
Method for preparation of large volume batches of poly-ICLC with increased biological potency; therapeutic, clinical and veterinary uses thereof | ONCOVIR, INC. | 1-Jul-
03 |
1-
Jul- 03 |
8-
Jan- 04 |
| US20030224
353 |
Antisense antiviral agent and method for treating ssRNA viral infection | IVERSEN PATRICK L. | 24-
Apr- 03 |
24-
Apr -03 |
4-
Dec -03 |
| US10669245 | Certain (2S)-N-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2- carboxamides dipeptidyl peptidase 1 inhibitors | ASTRAZENECA AB | 24-
Jan- 14 |
19-
Mar -19 |
2-
Jun- 20 |
| US10666592 | RNA targeting methods and compositions | Salk Institute for Biological Studies | 22-
Aug- 17 |
31-
Dec -18 |
26-
May -20 |
| US10662485 | Bioagent detection oligonucleotides | IBIS BIOSCIENCES, INC. | 27-
Dec- 11 |
13-
Apr -18 |
26-
May -20 |
| US10662464 | Methods of analyzing virus-derived therapeutics | American International Biotechnology, LLC | 31-
Jul-15 |
29-
Jul- 16 |
26-
May -20 |
| US10662423 | Compositions for and methods of identifying antigens | President and Fellows of Harvard College | 21-
Feb- 06 |
12-
Feb -18 |
26-
May -20 |
| US10660824 | Devices, system and method to control the delivery of oral medications to ensure they are efficacious , taken as prescribed, and to avoid unwanted side effects | Not Available | 11-
Aug- 15 |
11-
Aug -16 |
26-
May -20 |
| US10655108 | Cell-derived viral vaccines with low levels of residual cell DNA | Seqirus UK Limited | 1-
Nov- 05 |
1-
Nov -06 |
19-
May -20 |
| US10655099 | Animal protein-free media for cultivation of cells | Baxalta GmbH | 29-
Oct- 04 |
29-
Oct -18 |
19-
May -20 |
| US10654898 | Recombinant human/bovine parainfluenza virus 3 (B/HPIV3) expressing a chimeric RSV/BPIV3 F protein and uses thereof | The United States of America, as represented by the Secretary, Department of Health and Human Serices | 20-
Jan- 15 |
20-
Jan -16 |
19-
May -20 |
| US10647998 | Tissue preferential codon modified expression cassettes, vectors containing same, and uses thereof | The Trustees of the University of Pennsylvania | 29-
Apr- 13 |
20-
Jun -17 |
12-
May -20 |
| US10647781 | Generation of binding molecules | Merus N.V. | 26-
Sep- 11 |
22-
Nov -17 |
12-
May -20 |
| US10647758 | Compositions comprising AAV expressing dual antibody constructs and uses thereof | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | 13-
May- 14 |
2-
Jul- 19 |
12-
May -20 |
| US10646563 | Vaccines and immunotherapeutics using IL-28 and compositions and methods of using | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | 4-
Apr- 08 |
11-
Sep -18 |
12-
May -20 |
| US10646438 | Methods for inducing an immune response via buccal and/or sublingual administration of a vaccine | BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM | 26-
Jul-10 |
10-
May -17 |
12-
May -20 |
| US10641707 | Systems and methods for distinguishing optical signals of different modulation frequencies in an optical signal detector | Gen-Probe Incorporated | 24-
Feb- 11 |
21-
Mar -14 |
5-
May -20 |
| US10640788 | CRISPR-related methods and compositions with governing gRNAs | Editas Medicine, Inc. | 7-
Nov- 13 |
24-
Jan -19 |
5-
May -20 |
| US10640785 | Virus vectors for highly efficient transgene delivery | The Children’s Hospital of Philadelphia | 22-
Nov- 11 |
21-
Nov -12 |
5-
May -20 |
| US10640776 | Method for propagating adenoviral vectors encoding inhibitory gene products | GenVec, Inc. | 10-
Nov- 05 |
7-
Sep -17 |
5-
May -20 |
| US10640763 | Molecular indexing of internal sequences | Cellular Research, Inc. | 31-
May- 16 |
16-
May -17 |
5-
May -20 |
| US10633447 | Soluble engineered monomeric Fc | The United States of America, as represented by the Secretary, Department of Health and Human Services | 16-
Mar- 12 |
9-
May -17 |
28-
Apr- 20 |
| US10632133 | Anti-viral azide containing compounds | LIFE TECHNOLOGIES CORPORATION | 28-
Jul-10 |
7-
Dec -17 |
28-
Apr- 20 |
| US10626415 | Method of increasing the function of an AAV vector | The Trustees of the University of Pennsylvania | 7-
Apr- 05 |
27-
Oct -17 |
21-
Apr- 20 |
| US10626379 | Production of viruses in cell culture | Commonwealth Scientific and Industrial Research Organisation | 24-
Nov- 15 |
25-
Jan -19 |
21-
Apr- 20 |
| US10619186 | Methods and compositions for library normalization | Cellular Research, Inc. | 11-
Sep- 15 |
8-
Sep -16 |
14-
Apr- 20 |
| US10619153 | TAL effector-mediated DNA modification | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
5-
Oct -17 |
14-
Apr- 20 |
| US10617677 | Nuclear transport modulators and uses thereof | Biogen MA Inc. | 9-
May- 12 |
17-
Jul- 18 |
14-
Apr- 20 |
| US10614284 | Descriptive measurements and quantification of staining artifacts for in situ hybridization | Ventana Medical Systems, Inc. | 26-
May- 15 |
21-
Nov -17 |
7-
Apr- 20 |
| US10611827 | Non-human primate-derived pan-ebola and pan-filovirus monoclonal antibodies directed against envelope glycoproteins | INTEGRATED BIOTHERAPEUTICS, INC. | 28-
Oct- 14 |
27-
Oct -15 |
7-
Apr- 20 |
| US10610584 | Reverse genetics systems | Seqirus UK Limited | 31-
Jul-09 |
16-
Oct -17 |
7-
Apr- 20 |
| US10610571 | Cytokine conjugates for the treatment of proliferative and infectious diseases | SYNTHORX, INC. | 3-
Aug- 17 |
7-
Jun -19 |
7-
Apr- 20 |
| US10605808 | Antibody producing non-human animals | Merus N.V. | 27-
Jun- 08 |
27-
Apr -16 |
31-
Mar -20 |
| US10604729 | Liquid loading composition, method of making and use thereof | DEVMAR PRODUCTS, LLC | 8-
Sep- 16 |
21-
May -18 |
31-
Mar -20 |
| US10604574 | Oncolytic viral delivery of therapeutic polypeptides | ONCORUS, INC. | 30-
Jun- 16 |
25-
Oct -18 |
31-
Mar -20 |
| US10604561 | Anti-dengue virus antibodies, polypeptides containing variant Fc regions, and methods of use | Agency for Science, Technology and Research | 16-
Sep- 16 |
15-
Sep -17 |
31-
Mar -20 |
| US10604549 | Adenovirus comprising an albumin-binding moiety | FUNDACIA” INSTITUT D’INVESTIGACIA” BIOMA”DICA DE BELLVITGE (IDIBELL) | 30-
Apr- 14 |
30-
Apr -15 |
31-
Mar -20 |
| US10603356 | Compositions and method for treatment of inflammatory bowel disease | THE REGENTS OF THE UNIVERSITY OF CALIFORNIA | 2-
Jun- 11 |
21-
Apr -16 |
31-
Mar -20 |
| US10603299 | Prevention and treatment of viral infections | Not Available | 2-
Jun- 16 |
3-
Jul- 19 |
31-
Mar -20 |
| US10597736 | Compositions and methods for detecting viruses in a sample | Washington University | 29-
Jan- 16 |
27-
Jan -17 |
24-
Mar -20 |
| US10596264 | Peptides with viral infection enhancing properties and their use | Centre National de la Recherche Scientique | 30-
Jun- 11 |
28-
Jun -12 |
24-
Mar -20 |
| US10596197 | Red blood cell membrane-derived microparticles and their use for the treatment of lung disease | University of Pittburgh—Of the Commonwealth System of Higher Education | 7-
Nov- 13 |
1-
Jun -18 |
24-
Mar -20 |
| US10591714 | Endoscopic apparatus for thermal distribution monitoring | ELECTRONICS AND TELECOMMUNICATIONS RESEARCH INSTITUTE | 7-
Sep- 16 |
31-
May -17 |
17-
Mar -20 |
| US10590435 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
26-
Sep -18 |
17-
Mar -20 |
| US10590413 | Chiral control | WAVE LIFE SCIENCES LTD. | 13-
Jul-12 |
17-
Mar -17 |
17-
Mar -20 |
| US10590112 | Dihydropyrimidinyl benzazepine carboxamide compounds | Hoffmann-La Roche Inc. | 9-
Jun- 17 |
7-
Dec -18 |
17-
Mar -20 |
| US10588966 | Methods and compositions for inhibiting Akt3 | Augusta University Research Institute, Inc. | 15-
Jan- 16 |
6-
Feb -19 |
17-
Mar -20 |
| US10583086 | Technology for preparation of macromolecular microspheres | Ansun Biopharma, Inc. | 24-
Jan- 06 |
25-
Sep -17 |
10-
Mar -20 |
| US10577375 | Derivatives of porphyrins, their process of preparation and their use for treating viral infections | CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE | 30-
Oct- 15 |
28-
Oct -16 |
3-
Mar -20 |
| US10570416 | TC-83-derived alphavirus vectors, particles and methods | ALPHAVAX, INC. | 18-
May- 04 |
11-
Aug -16 |
25-
Feb -20 |
| US10570209 | Methods for inducing or enhancing an immune response by administering agonistic glucocorticoid-induced TNFR-family-related receptor (GITR) antibodies | GITR, Inc. | 25-
Mar- 05 |
28-
Jun -18 |
25-
Feb -20 |
| US10564160 | Antibody-secreting cell assay | MABTECH AB | 28-
Aug- 08 |
28-
Aug -09 |
18-
Feb -20 |
| US10564152 | Method and device for detecting antigen-specific antibodies in a biological fluid sample by using neodymium magnets | The United States of America, as represented by the Secretary, Department of Health and Human Services | 1-
Sep- 15 |
8-
Aug -16 |
18-
Feb -20 |
| US10563224 | Replication defective adenovirus vector in vaccination | Etubics Corporation | 24-
Aug- 12 |
15-
Feb -17 |
18-
Feb -20 |
| US10563154 | Disinfecting aqueous foam, process for preparing same and use thereof | COMMISSARIAT A L’ENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES | 16-
Jun- 15 |
15-
Jun -16 |
18-
Feb -20 |
| US10562861 | Carboxylic acid compounds | Sumitomo Dainippon Pharma Co., Ltd. | 18-
May- 12 |
23-
Oct -18 |
18-
Feb -20 |
| US10561743 | AAV vectors targeted to the central nervous system | The University of North Carolina at Chapel Hill | 21-
Nov- 14 |
13-
Jun -19 |
18-
Feb -20 |
| US10561722 | Methods and compositions for enhancing immune responses | Bavarian Nordic A/S | 3-
Sep- 14 |
3-
Sep -15 |
18-
Feb -20 |
| US10561126 | Genetically modified non-human animals and methods of use thereof | Institute for Research in Biomedicine (IRB) | 13-
Apr- 15 |
16-
Apr -18 |
18-
Feb -20 |
| US10557136 | In vivo delivery of oligonucleotides | Oncolmmunin Inc. | 12-
Dec- 11 |
12-
Dec -12 |
11-
Feb -20 |
| US10557119 | Erythroid cells comprising phenylalanine ammonia lyase | RUBIUS THERAPEUTICS, INC. | 18-
Nov- 13 |
10-
May -19 |
11-
Feb -20 |
| US10555993 | Dimethyl fumarate and vaccination regimens | Biogen MA Inc. | 14-
Mar- 14 |
23-
Apr -19 |
11-
Feb -20 |
| US10550378 | Composition comprising a gene vector that selectively depletes P16 positive senescent cells | Kythera Biopharmaceuticals, Inc. | 17-
Apr- 12 |
10-
Jun -19 |
4-
Feb -20 |
| US10550174 | Amino acid sequences directed against envelope proteins of a virus and polypeptides comprising the same for the treatment of viral diseases | Ablynx N.V. | 5-
Jun- 08 |
17-
Oct -17 |
4-
Feb -20 |
| US10548971 | MERS-CoV vaccine | INOVIO PHARMACEUTICALS, INC. | 29-
Nov- 13 |
29-
Jun -18 |
4-
Feb -20 |
| US10548959 | Compositions and methods for modified dendrimer nanoparticle delivery | Massachusetts Institute of Technology | 23-
Sep- 16 |
23-
Sep -16 |
4-
Feb -20 |
| US10544405 | Cas9-nucleic acid complexes and uses related thereto | Emory University | 16-
Jan- 13 |
15-
Jan -14 |
28-
Jan- 20 |
| US10544399 | Highly efficient influenza matrix (M1) proteins | Novavax, Inc. | 11-
Jul-03 |
22-
Mar -18 |
28-
Jan- 20 |
| US10544193 | Compositions and methods for treating diseases by inhibiting exosome release | MOREHOUSE SCHOOL OF MEDICINE | 19-
Dec- 16 |
9-
Jul- 18 |
28-
Jan- 20 |
| US10544108 | Hydrazide containing nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 29-
Jul-11 |
28-
Nov -18 |
28-
Jan- 20 |
| US10544102 | Benzazepine dicarboxamide compounds with secondary amide function | Hoffmann-La Roche Inc. | 19-
May- 17 |
13-
Nov -18 |
28-
Jan- 20 |
| US10543485 | Slip chip device and methods | University of Chicago | 24-
Mar- 09 |
25-
May -16 |
28-
Jan- 20 |
| US10543269 | hMPV RNA vaccines | ModernaTX, Inc. | 22-
Oct- 15 |
28-
Mar -19 |
28-
Jan- 20 |
| US10539488 | Sample fixation and stabilisation | RNASSIST LTD. | 1-
Mar- 13 |
14-
Aug -17 |
21-
Jan- 20 |
| US10538558 | Inhibition of TCR signaling with peptide variants | SIGNABLOK, INC | 30-
Sep- 09 |
22-
Oct -18 |
21-
Jan- 20 |
| US10538554 | Peptides and uses therefor as antiviral agents | VIRAMATIX SDN BHD | 27-
Nov- 15 |
28-
Nov -16 |
21-
Jan- 20 |
| US10533021 | Boron-containing small molecules | Anacor Pharmaceuticals, Inc. | 20-
Jun- 07 |
28-
Sep -17 |
14-
Jan- 20 |
| US10532111 | Recombinant adeno-associated virus capsids resistant to preexisting human neutralizing antibodies | The Board of Trustees of the Leland Stanford Junior University | 16-
Feb- 16 |
18-
Aug -17 |
14-
Jan- 20 |
| US10532110 | AAV vectors targeted to the central nervous system | The University of North Carolina at Chapel Hill | 21-
Nov- 14 |
20-
Nov -15 |
14-
Jan- 20 |
| US10532107 | Modified virus-like particles of CMV | SAIBA GMBH | 22-
Oct- 14 |
20-
Oct -15 |
14-
Jan- 20 |
| US10532067 | Delivery of RNA to trigger multiple immune pathways | GlaxoSmithKline Biologicals S.A. | 6-Jul-
10 |
5-
Oct -17 |
14-
Jan- 20 |
| US10527551 | Method of predicting a performance characteristic of a plant or yeast hydrolysate and its use | Baxalta GmbH | 30-
Dec- 13 |
18-
Dec -14 |
7-
Jan- 20 |
| US10526596 | Purification of nucleic acids using metal-titanium oxides | Abbott Molecular Inc. | 14-
Jul-15 |
5-
Jul- 19 |
7-
Jan- 20 |
| US10526295 | Nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 31-
Dec- 15 |
30-
Dec -16 |
7-
Jan- 20 |
| US10526292 | Dendrimer like amino amides possessing sodium channel blocker activity for the treatment of dry eye and other mucosal diseases | Parion Sciences, Inc. | 29-
May- 12 |
20-
Dec -17 |
7-
Jan- 20 |
| US10526283 | Prodrugs of dithiol mucolytic agents | PARION SCIENCES, INC. | 30-
Apr- 15 |
2-
May -16 |
7-
Jan- 20 |
| US10525120 | Methods and compositions for live attenuated viruses | TAKEDA VACCINES, INC. | 6-
Apr- 07 |
22-
Jul- 16 |
7-
Jan- 20 |
| US10525049 | Specific Akt3 inhibitor and uses thereof | Augusta University Research Institute, Inc. | 15-
Jan- 16 |
20-
May -19 |
7-
Jan- 20 |
| US10519130 | Quinazolinones and azaquinazolinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
2-
Nov -18 |
31-
Dec -19 |
| US10519129 | Quinazolinones and azaquinazolinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
2-
Nov -18 |
31-
Dec -19 |
| US10519128 | Quinazolinones and azaquinazolinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
2-
Nov -18 |
31-
Dec -19 |
| US10519127 | Quinazolinones and azaquinazolinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
2-
Nov -18 |
31-
Dec -19 |
| US10517947 | Methods for preparing squalene | NOVARTIS AG | 12-
May- 10 |
14-
Dec -17 |
31-
Dec -19 |
| US10517923 | Immunosuppressive agents and their use in therapy | Norwegian University of Science and Technology | 6-
Nov- 13 |
6-
Nov -14 |
31-
Dec -19 |
| US10517881 | Pharmaceutical compositions and methods | POP TEST ONCOLOGY LLC | 3-
Aug- 15 |
8-
Jan -19 |
31-
Dec -19 |
| US10513508 | Quinazolinones and azaquinazolinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
2-
Nov -18 |
24-
Dec -19 |
| US10513507 | Quinazolinones and azaquinazolinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
11-
Dec -17 |
24-
Dec -19 |
| US10512684 | Methods and compositions for intra-nasal immunization with recombinant MVA encoding flagellin | Bavarian Nordic A/S | 26-
Sep- 14 |
25-
Sep -15 |
24-
Dec -19 |
| US10512669 | Blockade of inflammatory proteases with cyclic peptides | The Regents of the University of California | 2-
Jun- 11 |
1-
Jun -12 |
24-
Dec -19 |
| US10512665 | Methods and compositions related to inhibition of viral entry | UNIVERSITY OF UTAH RESEARCH FOUNDATION | 8-
Feb- 07 |
2-
Jun -16 |
24-
Dec -19 |
| US10508098 | Quinazolinones and azaquinazolinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
2-
Nov -18 |
17-
Dec -19 |
| US10507244 | Anti-TIGIT antigen-binding proteins and methods of use thereof | Potenza Therapeutics, Inc. | 1-
Oct- 15 |
19-
Jun -17 |
17-
Dec -19 |
| US10503347 | System and method for detecting, collecting, analyzing, and communicating event-related information | Georgetown University | 25-
Feb- 08 |
27-
Jul- 17 |
10-
Dec -19 |
| US10501733 | Polypeptide assemblies and methods for the production thereof | University of Utah Research Foundation | 27-
Feb- 15 |
29-
Feb -16 |
10-
Dec -19 |
| US10501527 | Mast cell stabilizers for treatment of hypercytokinemia and viral infection | Emergo Therapeutics, Inc. | 8-
Sep- 16 |
7-
Sep -17 |
10-
Dec -19 |
| US10501507 | Griffithsin mutants | The United States of America, as represented by the Secretary, Department of Health and Human Services | 10-
Feb- 15 |
10-
Feb -16 |
10-
Dec -19 |
| US10501412 | Conjugates of cell binding molecules with cytotoxic agents | HANGZHOU DAC BIOTECH CO., LTD. | 12-
Jul-12 |
12-
Jul- 12 |
10-
Dec -19 |
| US10500272 | Manufacture of surfactant-containing compositions with enhanced stability | NOVARTIS AG | 2-
Dec- 14 |
12-
Mar -19 |
10-
Dec -19 |
| US10500267 | Influenza virus vectors and uses therefor | FluGen, Inc. | 17-
Mar- 14 |
21-
Aug -17 |
10-
Dec -19 |
| US10495640 | Exosome-mediated diagnosis of hepatitis virus infections and diseases | MOREHOUSE SCHOOL OF MEDICINE | 6-
Oct- 08 |
13-
Dec -16 |
3-
Dec -19 |
| US10494420 | Mast cell stabilizers for treatment of hypercytokinemia and viral infection | Emergo Therapeutics, Inc. | 8-
Sep- 16 |
15-
Nov -18 |
3-
Dec -19 |
| US10488353 | Apparatus and system for performing thermal melt analyses and a mplifications | GEN-PROBE INCORPORATED | 31-
Jul-12 |
23-
Feb -17 |
26-
Nov -19 |
| US10487350 | Methods for diagnosing infectious diseases using adsorption media | ExThera Medical Corporation | 8-
Nov- 13 |
16-
Oct -15 |
26-
Nov -19 |
| US10487332 | Immunisation of large mammals with low doses of RNA | GlaxoSmithKline Biologicals SA | 6-Jul-
10 |
6-
Jul- 11 |
26-
Nov -19 |
| US10487081 | Guanidine substituted imidazo[4,5-c] ring compounds | 3M Innovation Properties Company | 31-
Aug- 15 |
22-
Oct -18 |
26-
Nov -19 |
| US10485883 | Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 30-
Sep- 03 |
15-
Feb -17 |
26-
Nov -19 |
| US10485861 | Nanoparticle-based compositions | Massachusetts Institute of Technology | 14-
Mar- 13 |
14-
Mar -14 |
26-
Nov -19 |
| US10485856 | Carbon nanotube compositions and methods of use thereof | Yale University | 19-
Mar- 08 |
17-
Mar -17 |
26-
Nov -19 |
| US10485761 | Irradiated biodegradable polymer microparticles | GLAXOSMITHKLINE BIOLOGICALS, S.A. | 24-
Jan- 10 |
16-
May -18 |
26-
Nov -19 |
| US10479996 | Antisense antiviral compound and method for treating ss/RNA viral infection | Sarepta Therapeutics, Inc. | 16-
Sep- 04 |
6-
Nov -14 |
19-
Nov -19 |
| US10479781 | Peptidyl nitril compounds as dipeptidyl peptidase I inhibitors | Neuprozyme Therapeutics APS | 5-
Mar- 15 |
4-
Mar -16 |
19-
Nov -19 |
| US10476825 | RNA targeting methods and compositions | Salk Institue for Biological Studies | 22-
Aug- 17 |
27-
Mar -18 |
12-
Nov -19 |
| US10472647 | Primary mesenchymal stem cells as a vaccine platform | The Administrators of the Tulane Educational Fund | 21-
Dec- 12 |
15-
Nov -13 |
12-
Nov -19 |
| US10472420 | Immune response modifier conjugates | 3M Innovative Properties Company | 22-
Feb- 06 |
20-
Jan -15 |
12-
Nov -19 |
| US10472332 | Antiviral compounds and methods | Biotron Limited | 26-
Jun- 03 |
23-
May -17 |
12-
Nov -19 |
| US10471408 | Microspotting device | BioFire Diagnostics, LLC | 18-
Apr- 12 |
5-
Jan -17 |
12-
Nov -19 |
| US10471141 | Bisphosphonate-containing vaccine pharmaceutical composition for humoral immunity | NITTO DENKO CORPORATION | 3-
Sep- 14 |
2-
Sep -15 |
12-
Nov -19 |
| US10471140 | Composition for enhancing induction of humoral immunity, and vaccine pharmaceutical composition | NOTTO DENKO CORPORATION | 4-
Aug- 14 |
18-
Apr -19 |
12-
Nov -19 |
| US10471063 | Drug combination of PDE3/PDE4 inhibitor and muscarinic receptor antagonist | Verona Pharma PLC | 15-
Mar- 13 |
5-
Jun -17 |
12-
Nov -19 |
| US10466245 | Covalently linked thermostable kinase for decontamination process validation | The Secretary of State for Health | 20-
Feb- 08 |
8-
Jul- 16 |
5-
Nov -19 |
| US10464988 | Antibody/T-cell receptor chimeric constructs and uses thereof | EUREKA THERAPEUTICS, INC. | 23-
Oct- 15 |
4-
Sep -18 |
5-
Nov -19 |
| US10464975 | Stabilized anti-microbial peptides | Dana-Farber Cancer Institute, Inc. | 2-Jul-
15 |
1-
Jul- 16 |
5-
Nov -19 |
| US10464955 | Charged linkers and their uses for conjugation | HANGZHOU DAC BIOTECH CO., LTD. | 28-
Feb- 14 |
28-
Feb -14 |
5-
Nov -19 |
| US10464060 | Loading vials | BioFare Diagnostics, LLC | 10-
Nov- 11 |
9-
Nov -12 |
5-
Nov -19 |
| US10463723 | Methods and compositions for intranasal delivery | Shin Nippon Biomedical Laboratories, Ltd. | 15-
Apr- 10 |
14-
Mar -13 |
5-
Nov -19 |
| US10463615 | Circulation of components during microfluidization and/or homogenization of emulsions | NOVARTIS AG | 3-
Dec- 09 |
26-
Jun -17 |
5-
Nov -19 |
| US10457974 | Methods for diagnosing infectious diseases using adsorption media | ExThera Medical Corporation | 8-
Nov- 13 |
2-
Nov -16 |
29-
Oct- 19 |
| US10457901 | Cleaning composition, method of making and use thereof | DevMar Products, LLC | 8-
Sep- 16 |
19-
Sep -18 |
29-
Oct- 19 |
| US10456464 | Liquid immunity induction-promoting composition and vaccine pharmaceutical composition that include thrombosis treatment drug | NITTO DENKO CORPORATION | 4-
Aug- 14 |
4-
Aug -15 |
29-
Oct- 19 |
| US10450620 | Cell-free nucleic acids for the analysis of the human microbiome and components thereof | The Board of Trustees of the Leland Stanford Junior University | 7-
Nov- 13 |
7-
Nov -14 |
22-
Oct- 19 |
| US10450383 | Carbonic anhydrase IX (G250) antibodies and methods of use thereof | DANA-FARBER CANCER INSTITUTE, INC. | 2-
Dec- 05 |
9-
May -17 |
22-
Oct- 19 |
| US10443049 | Active low molecular weight variants of angiotensin converting enzyme 2 (ACE2) | Northwestern University | 24-
Jan- 17 |
24-
Jan -18 |
15-
Oct- 19 |
| US10442853 | Antibodies and processes for preparing the same | TAIGA BIOTECHNOLOGIES, INC. | 16-
May- 08 |
23-
Aug -16 |
15-
Oct- 19 |
| US10434158 | Combination of vaccination and inhibition of the PD-1 pathway | CureVac AG | 22-
Feb- 13 |
7-
Feb -18 |
8-
Oct- 19 |
| US10434116 | Methods of treating coronavirus infection | United States Government as represented by the Secretary, Department of Health and Human Services | 7-
Apr- 14 |
7-
Apr -15 |
8-
Oct- 19 |
| US10428128 | Helix-grafted proteins as inhibitors of disease-relevant proteinprotein interactions | Colorado State University Research Foundation | 7-
Nov- 14 |
9-
Nov -15 |
1-
Oct- 19 |
| US10428102 | Glycolipids and pharmaceutical compositions thereof for use in therapy | THE UNIVERSITY OF NOTTINGHAM | 4-
Apr- 14 |
7-
Apr -15 |
1-
Oct- 19 |
| US10428083 | Heterocyclylmethyl-thienouracile as antagonists of the adenosine- A2B-receptor | BAYER PHARMA AKTIENGESELLSCHAFT | 26-
Mar- 15 |
21-
Mar -16 |
1-
Oct- 19 |
| US10428027 | Sulfinylphenyl or sulfonimidoylphenyl benzazepines | Hoffmann La-Roche Inc. | 17-
Sep- 15 |
8-
Mar -18 |
1-
Oct- 19 |
| US10426737 | Lipids and lipid compositions for the delivery of active agents | Novartis AG | 19-
Dec- 13 |
17-
Dec -14 |
1-
Oct- 19 |
| US10421991 | Rapid epidemiologic typing of bacteria | BioFire Diagnostics, LLC | 19-
May- 08 |
11-
Nov -15 |
24-
Sep -19 |
| US10421962 | Double-stranded oligonucleotide molecules to DDIT4 and methods of use thereof | Quark Pharmaceuticals, Inc. | 12-
Sep- 12 |
15-
Feb -17 |
24-
Sep -19 |
| US10420837 | Vaccine pharmaceutical composition for transdermal administration | NITTO DENKO CORPORATION | 2-
Oct- 14 |
1-
Oct -15 |
24-
Sep -19 |
| US10420685 | Mobile clinics | Baylor College of Medicine | 12-
Nov- 14 |
11-
Nov -15 |
24-
Sep -19 |
| US10416171 | Influenza potency assays | Seqirus UK Limited | 7-Jul-
15 |
7-
Jul- 16 |
17-
Sep -19 |
| US10416161 | Exosome-mediated diagnosis of hepatitis virus infections and diseases | MOREHOUSE SCHOOL OF MEDICINE | 6-
Oct- 08 |
20-
Sep -16 |
17-
Sep -19 |
| US10414800 | Methods for producing a depsipeptide | NovoBiotic Pharmaceuticals, LLC | 3-
Dec- 12 |
15-
Jun -16 |
17-
Sep -19 |
| US10414779 | Fused [1,2]imidazo[4,5-C] ring compounds substituted with guanidino groups | 3M Innovative Properties Company | 26-
Aug- 16 |
1-
Aug -17 |
17-
Sep -19 |
| US10407492 | Amino acid sequences directed against envelope proteins of a virus and polypeptides comprising the same for the treatment of viral diseases | Ablynx N.V. | 5-
Jun- 08 |
17-
Oct -17 |
10-
Sep -19 |
| US10407472 | Fusion proteins, recombinant bacteria, and methods for using recombinant bacteria | Spogen Biotech Inc. | 17-
Sep- 14 |
14-
Dec -17 |
10-
Sep -19 |
| US10407431 | Compounds and compositions as toll-like receptor 7 agonists | Novartis AG | 1-
May- 14 |
27-
Feb -18 |
10-
Sep -19 |
| US10407405 | Nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 21-
Jun- 13 |
17-
Jul- 17 |
10-
Sep -19 |
| US10406229 | Methods and compositions related to inhibition of viral entry | UNIVERSITY OF UTAH RESEARCH FOUNDATION | 28-
Mar- 11 |
2-
Mar -17 |
10-
Sep -19 |
| US10406177 | Modified cells and methods of therapy | Intima Bioscience, Inc. | 31-
Jul-15 |
29-
Aug -16 |
10-
Sep -19 |
| US10406142 | Hydrazino 1H-imidazoquinolin-4-amines and conjugates made therefrom | 3M lnnovative Properties Company | 3-
Jun- 11 |
6-
Mar -17 |
10-
Sep -19 |
| US10400274 | Fluorogenic probes and their use in quantitative detection of target RNA sequences | Jan Biotech, Inc. | 18-
Jun- 16 |
16-
Jun -17 |
3-
Sep -19 |
| US10400225 | TAL effector-mediated DNA modification | Iowa State University Research Foundation, Inc. | 10-
Dec- 09 |
21-
Aug -17 |
3-
Sep -19 |
| US10400024 | Cleavage and exchange of major histocompatibility complex ligands employing azobenzene-containing peptides | SANQUIN REAGENTS B.V. | 6-
Jun- 14 |
5-
Jun -15 |
3-
Sep -19 |
| US10399963 | Substituted benzofuranyl and benzoxazolyl compounds and uses thereof | Karyopharm Therapeutics Inc. | 3-Jul-
13 |
4-
Dec -17 |
3-
Sep -19 |
| US10399941 | Conjugates of cell binding molecules with cytotoxic agents | HANGZHOU DAC BIOTECH CO., LTD. | 12-
Jul-12 |
16-
Apr -14 |
3-
Sep -19 |
| US10398795 | Decontamination device and method using ultrasonic cavitation | TOMI ENVIRONMENTAL SOLUTIONS, INC. | 29-
Dec- 17 |
29-
Dec -17 |
3-
Sep -19 |
| US10393633 | Sample fixation and stabilisation | RNASSIST LTD. | 1-
Mar- 13 |
30-
May -17 |
27-
Aug -19 |
| US10392613 | Purification of nucleic acids using copper-titanium oxides | ABBOTT MOLECULAR INC. | 14-
Jul-15 |
13-
Jul- 16 |
27-
Aug -19 |
| US10391188 | Decontamination device and method using ultrasonic cavitation | TOMI ENVIRONMENTAL SOLUTIONS, INC. | 29-
Dec- 17 |
11-
Sep -18 |
27-
Aug -19 |
| US10391167 | Mucosal vaccine composition | NITTO DENKO CORPORATION | 3-
Oct- 13 |
2-
Oct -14 |
27-
Aug -19 |
| US10391160 | Dimethyl fumarate and vaccination regimens | Biogen MA Inc. | 14-
Mar- 14 |
13-
Mar -15 |
27-
Aug -19 |
| US10385320 | Recombinant adeno-associated virus capsids with enhanced human skeletal muscle tropism | The Board of Trustees of the Leland Stanford Junior University | 2-
Dec- 15 |
2-
Dec -16 |
20-
Aug -19 |
| US10385119 | Compositions comprising AAV expressing dual antibody constructs and uses thereof | Trustees of the University of Pennsylvania | 13-
May- 14 |
15-
Oct -18 |
20-
Aug -19 |
| US10383938 | Lipidated immune response modifier compound compositions, formulations, and methods | 3M Innovative Properties Company | 17-
Aug- 10 |
24-
Jul- 18 |
20-
Aug -19 |
| US10383935 | Methods of making and using live attenuated viruses | Regents of the University of Minnesota | 23-
Sep- 15 |
31-
Oct -17 |
20-
Aug -19 |
| US10383852 | Prevention and treatment of viral infections | Not Available | 2-
Jun- 16 |
27-
Nov -18 |
20-
Aug -19 |
| US10378008 | Method and apparatus for automated processing of pooled samples | GFE BLUT MBH | 6-
Aug- 14 |
5-
Aug -15 |
13-
Aug -19 |
| US10378002 | Replication conditional virus that specifically kills senescent cells | Kythera Biopharmaceuticals, Inc. | 17-
Apr- 12 |
6-
Jul- 18 |
13-
Aug -19 |
| US10377773 | Isothiazolopyrimidinones, pyrazolopyrimidinones, and pyrrolopyrimidinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
27-
Feb -18 |
13-
Aug -19 |
| US10377767 | Thienopyrimidinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 5-
Feb- 15 |
1-
Mar -18 |
13-
Aug -19 |
| US10377760 | Pyrrolo and pyrazolopyrimidines as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 30-
Dec- 14 |
18-
Jan -18 |
13-
Aug -19 |
| US10370625 | Cleaning composition, method of making and use thereof | DEVMAR PRODUCTS, LLC | 8-
Sep- 16 |
6-
Sep -17 |
6-
Aug -19 |
| US10370455 | Identification of VSIG8 as the putative VISTA receptor (V-R) and use thereof to produce VISTA/VSIG8 agonists and antagonists | IMMUNEXT, INC. | 5-
Dec- 14 |
7-
Dec -15 |
6-
Aug -19 |
| US10370338 | Benzazepine dicarboxamide compounds with tertiary amide function | Hoffmann-La Roche Inc. | 23-
May- 16 |
13-
Nov -18 |
6-
Aug -19 |
| US10369219 | Composition for enhancing induction of humoral immunity, and vaccine pharmaceutical composition | NITTO DENKO CORPORATION | 4-
Aug- 14 |
4-
Aug -15 |
6-
Aug -19 |
| US10369216 | Polymeric carrier cargo complex for use as an immunostimulating agent or as an adjuvant | CureVac AG | 1-
Apr- 14 |
1-
Apr -15 |
6-
Aug -19 |
| US10369205 | Immunomodulatory compositions and methods of use thereof | Not Available | 7-
Jan- 14 |
7-
Jan -15 |
6-
Aug -19 |
| US10369204 | Molecular vaccines for infectious disease | Dako Denmark A/S | 2-
Oct- 08 |
2-
Oct -09 |
6-
Aug -19 |
| US10363303 | Microneedle compositions and methods of using same | VERNDARI, INC. | 11-
Jan- 16 |
11-
Jul- 18 |
30-
Jul- 19 |
| US10363282 | Analogs of C5a and methods of using same | Board of Regents of The University of Nebraska | 29-
Jun- 10 |
12-
Jan -18 |
30-
Jul- 19 |
| US10363247 | (S,E)-3-(6-aminopyridin-3-yl)-N-((5-(4-(3-fluoro-3- methylpyrrolidine-1-carbonyl)phenyl-7-(4-fluorophenyl)benzofuran- 2-yl)methyl)acrylamide for the treatment of cancer | Karyopharm Therapeutics Inc. | 18-
Aug- 15 |
18-
Aug -16 |
30-
Jul- 19 |
| US10358481 | Engineered antibody constant domain molecules | The United States of America, as represented by the Secretary, Department of Health and Human Services | 31-
Jan- 08 |
2-
Dec -16 |
23-
Jul- 19 |
| US10357568 | Adjuvant nanoemulsions with phospholipids | GLAXOSMITHKLINE BIOLOGICALS S.A. | 24-
Mar- 11 |
23-
Mar -12 |
23-
Jul- 19 |
| US10357562 | Immunoprotective primary mesenchymal stem cells and methods | Autoimmune Technologies, LLC | 14-
Mar- 13 |
17-
Jul- 15 |
23-
Jul- 19 |
| US10357510 | Metal nanoclusters and uses thereof | THE REGENTS OF THE UNIVERSITY OF MICHIGAN | 7-
Aug- 14 |
7-
Aug -15 |
23-
Jul- 19 |
| US10351571 | Pyrrolotriazinones and imidazotriazinones as ubiquitin-specific protease 7 inhibitors | FORMA Therapeutics, Inc. | 30-
Dec- 14 |
24-
May -18 |
16-
Jul- 19 |
| US10350255 | Polygonum cuspidatum extracts | PhotoDynamic Inc. | 1-
Oct- 15 |
30-
Sep -16 |
16-
Jul- 19 |
| US10344320 | Capacitive liquid crystal biosensors | The Johns Hopkins University | 6-
May- 15 |
5-
May -16 |
9-
Jul- 19 |
| US10344263 | Synthetic membrane-receiver complexes | RUBIUS THERAPEUTICS, INC. | 18-
Nov- 13 |
29-
Mar -17 |
9-
Jul- 19 |
| US10344261 | Immunomodulatory conjugates | ASCEND BIOPHARMACEUTICALS LTD | 9-
Nov- 11 |
9-
Nov -12 |
9-
Jul- 19 |
| US10344027 | Compositions and methods for inhibiting kinases | Inhibikase Therapeutics, Inc. | 23-
Apr- 15 |
24-
Oct -18 |
9-
Jul- 19 |
| US10342868 | Methods and compositions for inhibiting Akt3 | Augusta University Research Institute, Inc. | 15-
Jan- 16 |
17-
Jan -17 |
9-
Jul- 19 |
| US10342825 | Solution containing hypochlorous acid and methods of using same | Sonoma Pharmaceuticals, Inc. | 15-
Jun- 09 |
15-
Jun -10 |
9-
Jul- 19 |
| US10336725 | Chemical compounds | AstraZeneca AB | 18-
Mar- 14 |
13-
Dec -17 |
2-
Jul- 19 |
| US10335484 | Methods of generating robust passive and active immune responses | HUMABS BIOMED SA | 8-
Jan- 13 |
8-
Jan -14 |
2-
Jul- 19 |
| US10335393 | Nuclear transport modulators and uses thereof | Biogen MA Inc. | 9-
May- 12 |
4-
Dec -17 |
2-
Jul- 19 |
| US10335372 | Compositions with modified nucleases targeted to viral nucleic acids and methods of use for prevention and treatment of viral diseases | Jacob G. Appelbaum | 14-
Apr- 04 |
10-
Mar -17 |
2-
Jul- 19 |
| US10329531 | Synthetic membrane-receiver complexes | RUBIUS THERAPEUTICS, INC. | 18-
Nov- 13 |
13-
Oct -17 |
25-
Jun- 19 |
| US10329329 | Fusion proteins for promoting an immune response, nucleic acids encoding same, and methods of making and use thereof | UNIVERSITY OF MIAMI | 7-
Sep- 12 |
28-
Aug -18 |
25-
Jun- 19 |
| US10328157 | Acetylenedicarboxyl linkers and their uses in specific conjugation of a cell-binding molecule | HANGZHOU DAC BIOTECH CO., LTD. | 15-
Jul-15 |
3-
Feb -17 |
25-
Jun- 19 |
| US10323074 | Cryptic polypeptides and uses thereof | BOARD OF TRUSTEES OF MICHIGAN STATE UNIVERSITY | 29-
Jan- 15 |
12-
Dec -17 |
18-
Jun- 19 |
| US10322104 | Disulfur bridge linkers for conjugation of a cell-binding molecule | HANGZHOU DAC BIOTECH CO., LTD. | 15-
Jul-15 |
5-
Apr -17 |
18-
Jun- 19 |
| US10316031 | Compositions and methods for inhibiting kinases | Inhibikase Therapeutics, Inc. | 23-
Apr- 15 |
24-
Oct -18 |
11-
Jun- 19 |
| US10314893 | Oral delivery of angiotensin converting enzyme 2 (ACE2) or angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension, cardiac dysfunction and development of autoimmune and experimental induced ocular disorders | The Trustees of the University of Pennsylvania | 18-
Oct- 13 |
18-
Apr -16 |
11-
Jun- 19 |
| US10308913 | Chimeric viruses presenting non-native surface proteins and uses thereof | Icahn School of Medicine at Mount Sinai | 2-
Dec- 05 |
3-
Mar -16 |
4-
Jun- 19 |
| US10308705 | Optimized human clotting factor VIII gene expression cassettes and their use | The University of North Carolina at Chapel Hill | 6-
Feb- 15 |
5-
Feb -16 |
4-
Jun- 19 |
| US10308685 | Inhibitory peptides of viral infection | UNIVERSITY OF TENNESSEE RESEARCH FOUNDATION | 17-
Jul-14 |
25-
May -17 |
4-
Jun- 19 |
| US10307475 | Methods and compositions for immunization against virus | Academia Sinica | 27-
Mar- 09 |
18-
Feb -14 |
4-
Jun- 19 |
| US10307472 | Combination of vaccination and OX40 agonists | CureVac AG | 12-
Mar- 14 |
12-
Mar -14 |
4-
Jun- 19 |
| US10307439 | Substituted nucleosides, nucleotides and analogs thereof | Alios Biopharma, Inc. | 24-
Jun- 14 |
2-
Feb -17 |
4-
Jun- 19 |
| US10307434 | Nucleic acid prodrugs and methods of use thereof | WAVE LIFE SCIENCES LTD. | 6-Jul-
09 |
27-
May -16 |
4-
Jun- 19 |
| US10307391 | Disulfur bridge linkers for conjugation of a cell-binding molecule | HANGZHOU DAC BIOTECH CO., LTD. | 15-
Jul-15 |
5-
Apr -17 |
4-
Jun- 19 |
| US10307374 | Oil-in-water emulsions that contain nucleic acids | GLAXOSMITHKLINE BIOLOGICALS S.A. | 6-Jul-
11 |
24-
Apr -17 |
4-
Jun- 19 |
| US10301650 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
1-
May -17 |
28-
May -19 |
| US10301648 | Method of increasing the function of an AAV vector | The Trustees of the University of Pennsylvania | 7-
Apr- 05 |
18-
Feb -15 |
28-
May -19 |
| US10301594 | Synthetic membrane-receiver complexes | RUBIUS THERAPEUTICS, INC | 18-
Nov- 13 |
19-
Nov -18 |
28-
May -19 |
| US10301593 | Synthetic membrane-receiver complexes | RUBIUS THERAPEUTICS, INC. | 18-
Nov- 13 |
19-
Mar -18 |
28-
May -19 |
| US10301377 | Middle east respiratory syndrome coronavirus immunogens, antibodies, and their use | The United States of America, as Represented by the Secretary, Department of Health and Human Services | 24-
Feb- 15 |
24-
Feb -16 |
28-
May -19 |
| US10300149 | Compositions for enhancing transport of molecules into cells | SAREPTA THERAPEUTICS, INC. | 29-
Apr- 03 |
10-
Jan -17 |
28-
May -19 |
| US10300145 | Synthetic nanoparticles for delivery of immunomodulatory compounds | Massachusetts Institute of Technology | 15-
Jul-16 |
14-
Jul- 17 |
28-
May -19 |
| US10300127 | Immune complex | The Rockefeller University | 20-
Mar- 15 |
21-
Mar -16 |
28-
May -19 |
| US10300124 | Rodent hepadnavirus cores with reduced carrier-specific antigenicity | VLP BIOTECH, INC. | 15-
Mar- 13 |
14-
Mar -14 |
28-
May -19 |
| US10294534 | Respiratory infection assay | THE SECRETARY OF STATE FOR HEALTH | 9-
Dec- 11 |
10-
Dec -12 |
21-
May -19 |
| US10294293 | Human monoclonal antibody with specificity for dengue virus serotype 1 E protein and uses thereof | DSO National Laboratories | 14-
Dec- 10 |
1-
Jun -16 |
21-
May -19 |
| US10294280 | Constrained proteins and uses therefor | Monash University | 21-
Jul-14 |
21-
Jul- 15 |
21-
May -19 |
| US10293060 | Method for increasing expression of RNA-encoded proteins | CureVac AG | 21-
Aug- 14 |
19-
Feb -16 |
21-
May -19 |
| US10293055 | Acetylenedicarboxyl linkers and their uses in specific conjugation of a cell-binding molecule | HANGZHOU DAC BIOTECH CO., LTD. | 15-
Jul-15 |
3-
Mar -17 |
21-
May -19 |
| US10293039 | Attenuated Listeria monocytogenes mutant as a vaccine vector for the delivery of exogeneous antigens | Montana State University | 7-
Apr- 14 |
7-
Apr -15 |
21-
May -19 |
| US10292978 | Specific Akt3 inhibitor and uses thereof | Augusta University Research Institute, Inc. | 15-
Jan- 16 |
17-
Jan -17 |
21-
May -19 |
| US10292961 | Disulfur bridge linkers for conjugation of a cell-binding molecule | HANGZHOU DAC BIOTECH CO., LTD. | 15-
Jul-15 |
15-
Jul- 15 |
21-
May -19 |
| US10288601 | Method of determining, identifying or isolating cell-penetrating peptides | Phylogica Limited | 23-
May- 11 |
11-
Dec -17 |
14-
May -19 |
| US10287576 | Enzymatic encoding methods for efficient synthesis of large libraries | NUEVOLUTION A/S | 1-
Dec- 05 |
12-
Dec -16 |
14-
May -19 |
| US10287258 | Certain (2S)-N-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2- carboxamides as dipeptidyl peptidase 1 inhibitors | ASTRAZENECA AB | 24-
Jan- 14 |
19-
Sep -17 |
14-
May -19 |
| US10287253 | Substituted pyrimidines containing acidic groups as TLR7 modulators | APROS THERAPEUTICS, INC. | 5-
Dec- 16 |
4-
Dec -17 |
14-
May -19 |
| US10286067 | Composition for enhancing induction of humoral immunity, and vaccine pharmaceutical composition | NITTO DENKO CORPORATION | 4-
Aug- 14 |
4-
Aug -15 |
14-
May -19 |
| US10286056 | Adjuvant nanoemulsions with crystallisation inhibitors | GLAXOSMITHKLINE BIOLOGICALS S.A. | 27-
Jan- 11 |
27-
Jan -12 |
14-
May -19 |
| US10280199 | Coronavirus proteins and antigens | Phibro Animal Health Corporation | 7-
Feb- 14 |
4-
Aug -16 |
7-
May -19 |
| US10279029 | Immunogenic compositions and uses thereof | BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM | 25-
Mar- 15 |
27-
Feb -18 |
7-
May -19 |
| US10279028 | Compositions and methods for treating and preventing porcine reproductive and respiratory syndrome | Ohio State Innovation Foundation | 24-
Apr- 12 |
14-
Dec -17 |
7-
May -19 |
| US10279027 | Transgenic Vero-CD4/CCR5 cell line | INTERNATIONAL AIDS VACCINE INITIATIVE | 2-
Oct- 15 |
6-
Apr -17 |
7-
May -19 |
| US10273454 | Means and methods for influencing the stability of antibody producing cells | ACADEMISCH MEDISCH CENTRUM BIJ DE UNIVERSITEIT VAN AMSTERDAM | 9-
Dec- 05 |
2-
Jun -17 |
30-
Apr- 19 |
| US10273290 | Hydrocarbon double-stapled stabilized HIV-1 GP41 heptad repeat domain peptides | DANA-FARBER CANCER INSTITUTE, INC. | 18-
Jun- 09 |
21-
Sep -17 |
30-
Apr- 19 |
| US10272150 | Combination PIV3/hMPV RNA vaccines | ModernaTX, Inc. | 22-
Oct- 15 |
20-
Jul- 18 |
30-
Apr- 19 |
| US10272149 | Modified bat influenza viruses and their uses | J. CRAIG VENTER INSTITUTE | 5-
Sep- 14 |
4-
Sep -15 |
30-
Apr- 19 |
| US10266887 | CRISPR effector system based diagnostics | MASSACHUSETTS INSTITUTE OF TECHNOLOGY | 9-
Dec- 16 |
9-
Mar -18 |
23-
Apr- 19 |
| US10266886 | CRISPR effector system based diagnostics | MASSACHUSETTS INSTITUTED OF TECHNOLOGY | 9-
Dec- 16 |
9-
Mar -18 |
23-
Apr- 19 |
| US10266846 | Adeno-associated virus (AAV) serotype 8 sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 17-
Dec- 01 |
20-
Oct -16 |
23-
Apr- 19 |
| US10266545 | Coumarin derivative as antiviral agent, pharmaceutical composition thereof, its preparation and use | I-NOVA MEDICINSKA ISTRAZIVANJA D.O.O. | 2-
Feb- 16 |
2-
Feb -16 |
23-
Apr- 19 |
| US10265417 | Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor | The Trustees of the University of Pennsylvania | 30-
Sep- 03 |
3-
Aug -16 |
23-
Apr- 19 |
| US10265407 | Modular nanodevices for smart adaptable vaccines | Yale University | 15-
Feb- 07 |
10-
Nov -14 |
23-
Apr- 19 |
| US10265395 | Adjuvant compositions and related methods | Not Available | 24-
Mar- 15 |
23-
Mar -17 |
23-
Apr- 19 |
| US10265371 | Methods and reagents for efficient and targeted delivery of therapeutic molecules to CXCR4 cells | CENTRO DE INVESTIGACION BIOMEDICA EN RED EN BIOINGENIERIA BIOMATERIALES Y NANOMEDICINA (CIBER BBN) | 13-
Jan- 11 |
10-
Jan -17 |
23-
Apr- 19 |
| US10265291 | Disulfur bridge linkers for conjugation of a cell-binding molecule | HANGZHOU DAC BIOTECH CO., LTD. | 15-
Jul-15 |
13-
Feb -17 |
23-
Apr- 19 |
| US10260071 | CpG oligonucleotide analogs containing hydrophobic T analogs with enhanced immunostimulatory activity | COLEY PHARMACEUTICAL GMBH | 27-
Sep- 06 |
26-
May -16 |
16-
Apr- 19 |
| US10259865 | Anti-pneumococcal hyperimmune globulin for the treatment and prevention of pneumococcal infection | ADMA Biologics, Inc. | 15-
Mar- 17 |
15-
Mar -17 |
16-
Apr- 19 |
| US10259848 | Compositions and methods comprising hydrocarbon-stapled polypeptides | DANA-FARBER CANCER INSTITUTE, INC. | 23-
Jan- 08 |
4-
May -16 |
16-
Apr- 19 |
| US10258655 | Synergistic bacterial compositions and methods of production and use thereof | Seres Therapeutics, Inc. | 25-
Nov- 13 |
25-
Nov -14 |
16-
Apr- 19 |
| US10254204 | Membrane-assisted purification | Accelerate Diagnostics, Inc. | 7-
Mar- 11 |
3-
May -17 |
9-
Apr- 19 |
| US10253353 | Enhanced methods of ribonucleic acid hybridization | The Broad Institute, Inc. | 6-
Dec- 13 |
5-
Dec -14 |
9-
Apr- 19 |
| US10253318 | Methods and compositions for the treatment of cancer or other diseases | CITY OF HOPE | 26-
Jan- 07 |
14-
Jun -17 |
9-
Apr- 19 |
| US10253296 | Synthetic membrane-receiver complexes | RUBIUS THERAPEUTICS, INC. | 18-
Nov- 13 |
28-
Nov -17 |
9-
Apr- 19 |
| US10253093 | Human monoclonal antibodies against interleukin 8 (IL-8) | CORMORANT PHARMACEUTICALS AB | 16-
Dec- 02 |
30-
Apr -18 |
9-
Apr- 19 |
| US10251904 | Methods for treating arenaviridae and coronaviridae virus infections | GILEAD SCIENCES, INC. | 16-
Sep- 15 |
16-
Sep -16 |
9-
Apr- 19 |
| US10247729 | Media elaborated with newly synthesized antibodies (MENSA) and uses thereof | MICROBPLEX, INC. | 5-
May- 14 |
5-
May -15 |
2-
Apr- 19 |
| US10246425 | 3,5-diamino-6-chloro-N-(N-(4-phenylbutyl)carbamimidoyl) pyrazine-2-carboxamide compounds | Parion Sciences, Inc. | 17-
Dec- 12 |
29-
Jun -17 |
2-
Apr- 19 |
| US10238739 | Manufacture of surfactant-containing compositions with enhanced stability | NOVARTIS AG | 2-
Dec- 14 |
2-
Dec -15 |
26-
Mar -19 |
| US10238733 | Cationic oil-in-water emulsions | GLAXOSMITHKLINE BIOLOGICALS S.A. | 6-Jul-
10 |
11-
Mar -16 |
26-
Mar -19 |
| US10238666 | Pharmaceutical compositions and methods | Pop Test Oncology LLC | 3-
Aug- 15 |
29-
Nov -17 |
26-
Mar -19 |
| US10238633 | Methods for treating pulmonary emphysema using substituted 2- Aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)- amides inhibitors of Cathepsin C | Boehringer Ingelheim International GmbH | 14-
Mar- 13 |
6-
Jun -17 |
26-
Mar -19 |
| US10233429 | Hand, foot, and mouth vaccines and methods of manufacture and use thereof | Takeda Vaccines, Inc. | 7-
Nov- 14 |
27-
Oct -17 |
19-
Mar -19 |
| US10233425 | CD137 enrichment for efficient tumor infiltrating lymphocyte selection | The Trustees of the University of Pennsylvania | 16-
Sep- 13 |
16-
Sep -14 |
19-
Mar -19 |
| US10233237 | Heterodimeric immunoglobulins | AMGEN INC. | 21-
Nov- 12 |
21-
Nov -13 |
19-
Mar -19 |
| US10233158 | Arylalkyl- and aryloxyalkyl-substituted epithelial sodium channel blocking compounds | Parion Sciences, Inc. | 13-
Dec- 13 |
4-
Apr -18 |
19-
Mar -19 |
| US10232051 | Acetylenedicarboxyl linkers and their uses in specific conjugation of a cell-binding molecule | HANGZHOU DAC BIOTECH CO., LTD. | 15-
Jul-15 |
3-
Mar -17 |
19-
Mar -19 |
| US10227376 | Radiolabeled cationic steroid antimicrobials and diagnostic methods | BRIGHAM YOUNG UNIVERSITY | 22-
Aug- 14 |
19-
Aug -15 |
12-
Mar -19 |
| US10227373 | Enantiomers of the 1â€2,6â€2-isomer of neplanocin A | Auburn University | 4-
Aug- 14 |
17-
May -17 |
12-
Mar -19 |
| US10226449 | Heterocyclic modulators of lipid synthesis and combinations thereof | 3-V Biosciences, Inc. | 20-
Dec- 13 |
19-
Dec -14 |
12-
Mar -19 |
| US10226434 | Design, synthesis and methods of use of acyclic fleximer nucleoside analogues having anti-coronavirus activity | Katholieke Universiteit Leuven/Lieden University Medical Center, RC Leiden | 30-
Jan- 15 |
2-
Jul- 18 |
12-
Mar -19 |
| US10222374 | B-cell antigen presenting cell assay | Univeersity of Pittsburgh—Of the Commonwealth System of Higher Education | 8-
Apr- 10 |
9-
Aug -17 |
5-
Mar -19 |
| US10221446 | Signal propagation biomolecules, devices and methods | STC.UNM | 21-
May- 13 |
3-
Oct -16 |
5-
Mar -19 |
| US10220002 | Controlled-release peptide compositions and uses thereof | BOARD OF REGENTS OF THE UNIVERSITY OF NEBRASKA | 2-
Dec- 11 |
30-
Nov -12 |
5-
Mar -19 |
| US10213383 | Hydrophilic filtration during manufacture of vaccine adjuvants | NOVARTIS AG | 3-
Dec- 09 |
3-
Dec -10 |
26-
Feb -19 |
| US10209254 | Chips, detection systems, and methods for multiplex pneumococcus serology | The UAB Research Foundation | 9-
Oct- 13 |
9-
Oct -14 |
19-
Feb -19 |
| US10209248 | Multiplex immuno screening assay | Institut Pasteur | 4-
May- 12 |
19-
Jul- 17 |
19-
Feb -19 |
| US10206994 | RNA virus attenuation by alteration of mutational robustness and sequence space | INSTITUT PASTEUR | 28-
Jan- 15 |
28-
Jan -16 |
19-
Feb -19 |
| US10202640 | Single cell analysis of T cells using high-throughput multiplex amplification and deep sequencing | The Board of Trustees of the Leland Stanford Junior University | 7-
May- 14 |
30-
Apr -15 |
12-
Feb -19 |
| US10202617 | Expression cassette for efficient surface display of antigenic proteins | Temasek Life Sciences Laboratory Limited | 8-
Feb- 11 |
8-
Feb -12 |
12-
Feb -19 |
| US10202615 | Mammalian genes involved in toxicity and infection | VANDERBILT UNIVERSITY | 10-
Dec- 10 |
11-
Dec -11 |
12-
Feb -19 |
| US10202578 | Chicken cells for improved virus production | THE PIRBRIGHT INSTITUTE | 5-
Jun- 13 |
3-
Jun -14 |
12-
Feb -19 |
| US10202367 | Heterocyclic compounds and methods of use thereof | The United States of America, as represented by the Secretary, Department of Health and Human Services | 12-
Jun- 14 |
12-
Jun -15 |
12-
Feb -19 |
| US10201198 | Protective masks with coating comprising different electrospun fibers interweaved with each other, formulations forming the same, and method of producing thereof | Profit Royal Pharmaceutical Limited | 23-
Dec- 14 |
10-
Dec -15 |
12-
Feb -19 |
| US10190984 | Systems and methods for analyzing a sample and for monitoring the performance of an optical signal detector | GEN-PROBE INCORPORATED | 31-
Dec- 15 |
23-
Dec -16 |
29-
Jan- 19 |
| US10190137 | CRISPR-related methods and compositions with governing gRNAS | Editas Medicine, Inc. | 7-
Nov- 13 |
29-
Nov -17 |
29-
Jan- 19 |
| US10190132 | Recombinant influenza virus-like particles (VLPs) produced in transgenic plants expressing hemagglutinin | MEDICAGO INC. | 21-
Jan- 08 |
2-
Sep -16 |
29-
Jan- 19 |
| US10189822 | Heterocyclic modulators of lipid synthesis | 3-V Biosciences, Inc. | 19-
Mar- 15 |
15-
Mar -16 |
29-
Jan- 19 |
| US10189820 | Heterocyclic amides useful as protein modulators | GlaxoSmithKline Intellectual Property Development Limited | 7-
Apr- 16 |
3-
Jan -18 |
29-
Jan- 19 |
| US10183074 | Cationic oil-in-water emulsions | GLAXOSMITHKLINE BIOLOGICALS S.A. | 6-Jul-
11 |
23-
Mar -17 |
22-
Jan- 19 |
| US10179176 | Recombinant adeno-associated virus capsids resistant to preexisting human neutralizing antibodies | The Board of Trustees of the Leland Stanford Junior University | 16-
Feb- 16 |
16-
Feb -17 |
15-
Jan- 19 |
| US10179143 | Anti-viral azide containing compounds | Life Technologies Corporation | 28-
Jul-10 |
8-
Aug -17 |
15-
Jan- 19 |
| US10173987 | Hydrazide containing nuclear transport modulators and uses thereof | Karyopharm Therapeutics Inc. | 29-
Jul-11 |
21-
Jun -17 |
8-
Jan- 19 |
| US10172830 | Pyrazolone compounds having human neutrophil elastase inhibitory properties | Chiesi Farmaceutici S.p.A. | 31-
May- 16 |
26-
May -17 |
8-
Jan- 19 |
| US10168336 | Quinone methide analog signal amplification | Ventana Medical Systems, Inc. | 24-
Feb- 14 |
24-
Aug -16 |
1-
Jan- 19 |
| US10167499 | Luminophore-labeled molecules coupled with particles for microarray-based assays | CAPITALBIO TECHNOLOOGY CORPORATION | 5-
Dec- 13 |
2-
Dec -14 |
1-
Jan- 19 |
| US10167333 | Neutralizing human monoclonal antibodies against hepatitis B virus surface antigen | Not Available | 16-
Jan- 14 |
15-
Jan -15 |
1-
Jan- 19 |
| US10166283 | Nucleic acid comprising or coding for a histone stem-loop and a poly(A) sequence or a polyadenylation signal for increasing the expression of an encoded pathogenic antigen | CureVac AG | 15-
Feb- 12 |
21-
Mar -17 |
1-
Jan- 19 |
| US10166255 | Intracellular genomic transplant and methods of therapy | INTIMA BIOSCIENCE, INC. | 31-
Jul-15 |
29-
Jul- 16 |
1-
Jan- 19 |
| US10160796 | Mast cell stabilizers for treatment of hypercytokinemia and viral infection | Emergo Therapeutics, Inc. | 8-
Sep- 16 |
17-
Nov -17 |
25-
Dec -18 |
| US10159731 | Methods and compositions for inhibiting Akt3 | Augusta University Research Institute, Inc. | 15-
Jan- 16 |
20-
Feb -18 |
25-
Dec -18 |
| US10159729 | Antigen and method for production thereof | Sallpro Biotech AB | 13-
Sep- 13 |
12-
Sep -14 |
25-
Dec -18 |
| US10159672 | Chemically and metabolically stable dipeptide possessing potent sodium channel blocker activity | PARION SCIENCES, INC. | 27-
Jun- 11 |
15-
Dec -17 |
25-
Dec -18 |
| US10156562 | Assay for detecting Th1 and Th2 cell populations | AMGEN INC. | 16-
May- 14 |
15-
May -15 |
18-
Dec -18 |
| US10155980 | Compositions and methods for detecting rare sequence variants | ACCURAGEN HOLDINGS LIMITED | 15-
Aug- 16 |
1-
Nov -17 |
18-
Dec -18 |
| US10155946 | Particle-nucleic acid conjugates and therapeutic uses related thereto | Emory University | 25-
Jun- 12 |
9-
Oct -17 |
18-
Dec -18 |
| US10155932 | Decreasing potential iatrogenic risks associated with influenza vaccines | Novartis AG | 9-
Sep- 04 |
9-
May -16 |
18-
Dec -18 |
| US10150768 | Guanidine substituted imidazo[4,5-c] ring compounds | 3M Innovative Properties Company | 31-
Aug- 15 |
26-
Aug -16 |
11-
Dec -18 |
| US10150743 | Carboxylic acid compounds | Sumitomo Dainippon Pharma Co., Ltd. | 18-
May- 12 |
11-
May -16 |
11-
Dec -18 |
| US10149901 | Influenza vaccines with reduced amounts of squalene | Seqirus UK Limited | 10-
Feb- 09 |
27-
Jan -16 |
11-
Dec -18 |
| US10149859 | Nucleotide and nucleoside therapeutic compositions and uses related thereto | Emory University | 11-
Sep- 13 |
10-
Sep -14 |
11-
Dec -18 |
| US10149461 | Immunocompromised ungulates | Revivicor, Inc. | 27-
Oct- 08 |
23-
Mar -15 |
11-
Dec -18 |
| US10144735 | Immune response modifier compositions and methods | 3M Innovative Properties Company | 22-
Dec- 06 |
25-
Jun -18 |
4-
Dec -18 |
| US10143709 | Use of ASC and ASC-CM to treat ARDS, SARS, and MERS | Indiana University Research and Technology Corporation | 6-
May- 14 |
6-
May -15 |
4-
Dec -18 |
| US10143652 | Methods for the preparation of liposomes | CuriRx Inc. | 23-
Sep- 09 |
2-
Aug -16 |
4-
Dec -18 |
| US10138461 | Animal protein-free media for cultivation of cells | Baxalta Gmbh | 29-
Oct- 04 |
22-
Sep -17 |
27-
Nov -18 |
| US10138295 | Compositions comprising AAV expressing dual antibody constructs and uses thereof | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | 13-
May- 14 |
13-
May -15 |
27-
Nov -18 |
| US10138276 | Inhibition of TCR signaling with peptide variants | SIGNABLOK, Inc. | 30-
Sep- 09 |
30-
Sep -10 |
27-
Nov -18 |
| US10131709 | Nucleic acid molecules encoding monoclonal antibodies specific for IL-22 | ImmunoQure AG | 28-
Dec- 11 |
7-
Sep -16 |
20-
Nov -18 |
| US10131704 | Middle east respiratory syndrome coronavirus neutralizing antibodies and methods of use thereof | DANA-FARBER CANCER INSTITUTE, INC. | 25-
Apr- 14 |
27-
Apr -15 |
20-
Nov -18 |
| US10131682 | Hydrophilic linkers and their uses for conjugation of drugs to a cell binding molecules | HANGZHOU DAC BIOTECH CO., LTD. | 24-
Nov- 12 |
24-
Nov -12 |
20-
Nov -18 |
| US10130701 | Coronavirus | THE PIRBRIGHT INSTITUTE | 23-
Jul-14 |
23-
Jul- 15 |
20-
Nov -18 |
| US10125112 | Modulators of the relaxin receptor 1 | THE FLORIDA INTERNATIONAL UNIVERSITY BOARD OF TRUSTEES | 4-
May- 12 |
25-
Aug -16 |
13-
Nov -18 |
| US10125092 | Lipids and lipid compositions for the delivery of active agents | Novartis AG | 5-
Sep- 14 |
4-
Sep -15 |
13-
Nov -18 |
| US10124065 | Lipids and lipid compositions for the delivery of active agents | Novartis AG | 8-
Mar- 13 |
6-
Mar -14 |
13-
Nov -18 |
| US10124048 | Adenovirus vectors | Oxford University Innovation Limited | 10-
Apr- 07 |
27-
Apr -15 |
13-
Nov -18 |
| US10123518 | Genetically modified non-human animals and methods of use thereof | Institute For Research In Biomedicine (IRB) | 13-
Apr- 15 |
12-
Apr -16 |
13-
Nov -18 |
| US10119967 | Multiplex immuno screening assay | Institut Pasteur | 4-
May- 12 |
3-
May -13 |
6-
Nov -18 |
| US10119164 | Capture primers and capture sequence linked solid supports for molecular diagnostic tests | IBIS BIOSCIENCES, INC. | 31-
Jul-09 |
15-
Aug -16 |
6-
Nov -18 |
| US10118925 | Imidazo[4,5-c] ring compounds containing substituted guanidine groups | 3M Innovative Properties Company | 31-
Aug- 15 |
26-
Aug -16 |
6-
Nov -18 |
| US10118923 | Compositions and methods for inhibiting kinases | Inhibikase Therapeutics, Inc. | 23-
Apr- 15 |
7-
Nov -17 |
6-
Nov -18 |
| US10117920 | Combination of vaccination and inhibition of the PD-1 pathway | CureVac AG | 22-
Feb- 13 |
7-
Feb -18 |
6-
Nov -18 |
| US10114011 | Antigen presenting cell assay | University of Pittsburgha€”Of the Commonwealth System of Higher Education | 8-
Apr- 10 |
20-
Jul- 17 |
30-
Oct- 18 |
| US10106619 | Virus vaccination and treatment methods with OX40 agonist compositions | La Jolla Institute for Allergy and Immunology | 4-
Oct- 06 |
4-
Oct -07 |
23-
Oct- 18 |
| US10106551 | Monothiol mucolytic agents | PARION SCIENCES, INC. | 30-
Jan- 15 |
29-
Jan -16 |
23-
Oct- 18 |
| US10105426 | Immunostimulatory combinations | TRUSTEES OF DARTMOUTH COLLEGE | 30-
Dec- 02 |
10-
Sep -15 |
23-
Oct- 18 |
| US10078083 | Detecting targets using mass tags and mass spectrometry | Ventana Medical Systems, Inc. | 2-Jul-
10 |
28-
Dec -15 |
18-
Sep -18 |
| US10077427 | Means and methods for influencing the stability of cells | ACADEMISCH MEDISCH CENTRUM BIJ DE UNIVERSITEIT VAN AMSTERDAM | 26-
Mar- 15 |
26-
Mar -15 |
18-
Sep -18 |
| US10076491 | Vaccine composition | NITTO DENKO CORPORATION | 5-
Feb- 13 |
29-
Jan -14 |
18-
Sep -18 |
| US10072309 | Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids | Not Available | 8-
May- 15 |
6-
May -16 |
11-
Sep -18 |
| US10072064 | Composition comprised of antigen linked to a TNF superfamily ligand | Not Available | 15-
Mar- 13 |
16-
Mar -14 |
11-
Sep -18 |
| US10072058 | Chimeric virus-like particles incorporating fusion GPI anchored GM- CSF and IL-4 conjugates | Children’s Healthcare of Atlanta, Inc. | 29-
Apr- 15 |
29-
Apr -16 |
11-
Sep -18 |
| US10071976 | Small molecule fatty acid synthase inhibitors | SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE | 7-
Mar- 14 |
5-
Mar -15 |
11-
Sep -18 |
| US10071970 | Chloro-pyrazine carboxamide derivatives with epithelial sodium channel blocking activity | Parion Sciences, Inc. | 17-
Dec- 12 |
7-
Mar -17 |
11-
Sep -18 |
| US10071155 | Nasal mucosal vaccine composition | NITTO DENKO CORPORATION | 3-
Oct- 13 |
2-
Oct -14 |
11-
Sep -18 |
| US10071154 | Vaccines and immunotherapeutics using IL-28 and compositions and methods of using the same | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | 4-
Apr- 08 |
6-
Apr -09 |
11-
Sep -18 |
| US10071076 | Methods of treating cancer and other disorders | Wake Forest University Health Sciences | 12-
Nov- 10 |
27-
Jun -16 |
11-
Sep -18 |
| US10066238 | Methods for producing antibodies | UNIVERSITY OF LOUISVILLE RESEARCH FOUNDATION, INC. | 11-
Feb- 13 |
11-
Feb -14 |
4-
Sep -18 |
| US10066012 | Human monoclonal antibodies against interleukin 8 (IL-8) | CORMORANT PHARMACEUTICALS AB | 16-
Dec- 02 |
26-
Apr -16 |
4-
Sep -18 |
| US10064934 | Combination PIV3/hMPV RNA vaccines | ModernaTX, Inc. | 22-
Oct- 15 |
11-
Aug -17 |
4-
Sep -18 |
| US10064900 | Methods of populating a gastrointestinal tract | Seres Therapeutics, Inc. | 4-
Feb- 13 |
4-
Feb -14 |
4-
Sep -18 |
| US10059769 | Anti-PD-Ll antibodies and uses thereof | I-MAB | 13-
Jun- 16 |
13-
Jun -17 |
28-
Aug -18 |
| US10059741 | Peptidomimetic macrocycles | Aileron Therapeutics, Inc. | 1-Jul-
15 |
1-
Jul- 16 |
28-
Aug -18 |
| US10059655 | Lipids and lipid compositions for the delivery of active agents | Novartis AG | 19-
Dec- 13 |
17-
Dec -14 |
28-
Aug -18 |
| US10058624 | Recombinant promoters and vectors for protein expression in liver and use thereof | Children’s Healthcare of Atlanta, Inc. | 16-
Apr- 15 |
15-
Apr -16 |
28-
Aug -18 |
| US10058535 | Nuclear transport modulators and uses thereof | Biogen MA Inc. | 9-
May- 12 |
24-
Jan -17 |
28-
Aug -18 |
| US10058516 | Design, synthesis and methods of use of acyclic fleximer nucleoside analogues having anti-coronavirus activity | Katholieke Universiteit Leuven | 30-
Jan- 15 |
28-
Jan -16 |
28-
Aug -18 |
| US10055502 | System and method for detecting, collecting, analyzing, and communicating event related information | Georgetown University | 25-
Feb- 08 |
23-
Sep -16 |
21-
Aug -18 |
| US10053728 | High density self-contained biological analysis | BioFire Diagnostics, LLC | 15-
Nov- 06 |
16-
Jul- 15 |
21-
Aug -18 |
| US10052398 | Additive compositions for pigmented disinfection and methods thereof | Kinnos Inc. | 8-
Dec- 14 |
23-
May -15 |
21-
Aug -18 |
| US10052380 | Lipidated immune response modifier compound compositions, formulations, and methods | 3M Innovative Properties Company | 17-
Aug- 10 |
27-
Nov -17 |
21-
Aug -18 |
| US10047375 | Artificial nucleic acid molecules | CureVac AG | 30-
Dec- 14 |
28-
Jun -16 |
14-
Aug -18 |
| US10047148 | Neutralizing GP41 antibodies and their use | The United States of America, as represented by the Secretary, Department of Health and Human Services | 7-
Nov- 11 |
8-
Sep -17 |
14-
Aug -18 |
| US10047147 | Neutralizing GP41 antibodies and their use | The United States of American, as represented by the Secretary, Department of Health and Human Services | 7-
Nov- 11 |
4-
Aug -14 |
14-
Aug -18 |
| US10046048 | Homogenous suspension of immunopotentiating compounds and uses thereof | GLAXOSMITHKLINE BIOLOGICALS S.A. | 15-
Dec- 09 |
5-
Jul- 16 |
14-
Aug -18 |
| US10040831 | Compositions and methods for treating diseases by inhibiting exosome release | MOREHOUSE SCHOOL OF MEDICINE | 19-
Dec- 16 |
19-
Dec -16 |
7-
Aug -18 |
| US10040828 | Human respiratory syncytial virus consensus antigens, nucleic acid constructs and vaccines made therefrom, and methods of using same | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | 10-
Apr- 12 |
10-
Apr -13 |
7-
Aug -18 |
| US10040820 | Method for the purification of protein complexes | Immunobiology Limited | 19-
Jun- 09 |
21-
Jun -10 |
7-
Aug -18 |
| US10039781 | Pulse inhalation of nitric oxide for treating respiratory diseases | AIT THERAPEUTICS, INC. | 24-
Mar- 15 |
24-
Mar -16 |
7-
Aug -18 |
| US10039758 | Compositions and methods for inhibiting bacterial and viral pathogens | Keck Graduate Institute of Applied Life Sciences | 24-
Oct- 14 |
23-
Oct -15 |
7-
Aug -18 |
| US10034931 | Use of EGFR pathway inhibitors to increase immune responses to antigens | Emory University | 23-
Sep- 13 |
23-
Sep -14 |
31-
Jul- 18 |
| US10034894 | Method of treating inflammation | CYTOSORBENTS CORPORATION | 1-
Apr- 10 |
28-
Jun -17 |
31-
Jul- 18 |
| US10031134 | Antibody-nanoparticle conjugates and methods for making and using such conjugates | Ventana Medical Systems, Inc. | 27-
Apr- 10 |
12-
Sep -16 |
24-
Jul- 18 |
| US10030250 | Edible vaccines expressed in soybeans | Not Available | 12-
Oct- 04 |
25-
Jan -10 |
24-
Jul- 18 |
| US10030074 | Methods of inducing or enhancing an immune response in a subject having cancer by administering GITR antibodies | GITR, Inc. | 25-
Mar- 05 |
7-
Nov -16 |
24-
Jul- 18 |
| US10030053 | Immunogenic compositions and methods of use thereof | Emory University | 17-
Dec- 07 |
13-
Feb -15 |
24-
Jul- 18 |
| US10029016 | Immunostimulatory compositions and methods of use thereof | Massachusetts Insitute of Technology | 5-
Apr- 12 |
2-
Jul- 15 |
24-
Jul- 18 |
| US10028482 | Disinfecting and deodorizing compositions and methods with novel polymeric binding system | OxiScience LLC | 28-
Aug- 14 |
28-
Aug -15 |
24-
Jul- 18 |
| US10023845 | Methods of making modified viral genomes | The Research Foundation for The State University of New York | 30-
Mar- 07 |
7-
Sep -16 |
17-
Jul- 18 |
| US10023632 | Antigenic GM-CSF peptides and antibodies to GM-CSF | Morphotek, Inc. | 8-
Feb- 06 |
18-
Jul- 16 |
17-
Jul- 18 |
| US10023558 | Compounds | CHIESI FARMACEUTICI S.P.A. | 31-
May- 16 |
22-
Sep -17 |
17-
Jul- 18 |
| US10022436 | Microneedle compositions and methods of using same | VERNDARI, INC. | 11-
Jan- 16 |
11-
Jan -17 |
17-
Jul- 18 |
| US10022435 | Nucleic acid vaccines | ModernaTX, Inc. | 23-
Apr- 14 |
1-
Apr -16 |
17-
Jul- 18 |
| US10022422 | Peptidomimetic macrocycles | Alleron Therapeutics, Inc. | 14-
Jan- 09 |
7-
Apr -16 |
17-
Jul- 18 |
| US10018369 | Air curtain device | KAWANO GIKEN CO., LTD. | 7-
Aug- 15 |
19-
Nov -15 |
10-
Jul- 18 |
| US10017784 | Gene transfer into airway epithelial stem cell by using lentiviral vector pseudotyped with RNA virus or DNA virus spike protein | ID PHARMA CO., LTD. | 28-
Oct- 05 |
17-
Nov -14 |
10-
Jul- 18 |
| US10016498 | D-amino acid derivative-modified peptidoglycan and methods of use thereof | The Regents of the University of California | 30-
Nov- 12 |
14-
Sep -17 |
10-
Jul- 18 |
| US10016497 | MERS-CoV vaccine | INOVIO PHARMACEUTICALS, INC. | 29-
Nov- 13 |
26-
Nov -14 |
10-
Jul- 18 |
| US10016455 | Method of preventing or treating influenza with oxidative reductive potential water solution | Sonoma Pharmaceuticals, Inc. | 30-
Dec- 03 |
9-
May -17 |
10-
Jul- 18 |
| US10013760 | Stain-free histopathology by chemical imaging | BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS | 15-
Mar- 13 |
2-
Aug -17 |
3-
Jul- 18 |
| US10010718 | Device to kill micro-organisms inside the respiratory tract | Not Available | 1-
Apr- 16 |
28-
Mar -17 |
3-
Jul- 18 |
| US10010607 | Method for preparing viral particles with cyclic dinucleotide and use of said particles for inducing immune response | INSERM (INSTITUT NATIONAL DE LA SANTE ET DE LA RECHERCHE MEDICALE) | 16-
Sep- 14 |
16-
Sep -15 |
3-
Jul- 18 |
| US10006862 | Continuous process for performing multiple nucleic acid amplification assays | GEN-PROBE INCORPORATED | 10-
Mar- 05 |
19-
May -17 |
26-
Jun- 18 |
| US10005833 | Methods of treating inflammation associated airway diseases and viral infections | THE BOARD OF REGENTS OF THE UNIVERSITY OF TEXAS SYSTEM | 2-
Jan- 15 |
31-
Dec -15 |
26-
Jun- 18 |
| US10005772 | Immune response modifier compositions and methods | 3M Innovative Properties Company | 22-
Dec- 06 |
20-
Dec -07 |
26-
Jun- 18 |
| US10004764 | Red blood cell membrane-derived microparticles and their use for the treatment of lung disease | University of Pittsburgha€”Of the Commonwealth System of Higher Education | 7-
Nov- 13 |
6-
Nov -14 |
26-
Jun- 18 |
| US10004755 | Therapeutic uses of selected pyrrolopyrimidine compounds with anti-mer tyrosine kinase activity | The University of North Carolina at Chapel Hill | 11-
Apr- 14 |
30-
Jan -17 |
26-
Jun- 18 |
| USRE47838 | Inhibitory peptides of viral infection | UNIVERSITY OF TENNESSEE RESEARCH FOUNDATION | 17-
Jul-14 |
30-
Jan -18 |
4-
Feb -20 |
| USRE47636 | Substituted spirocycles | Boehringer Ingelheim International GmbH | 12-
Sep- 14 |
30-
Nov -17 |
8-
Oct- 19 |
| USRE47493 | Substituted bicyclic dihydropyrimidinones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelheim International GmbH | 20-
Feb- 14 |
26-
Mar -18 |
9-
Jul- 19 |
| USRE46906 | Methods for producing vaccine adjuvants | NOVARTIS AG | 3-
Dec- 09 |
13-
Oct -15 |
26-
Jun- 18 |
| USRE46873 | Multi-targeted RNAi therapeutics for scarless wound healing of skin | Sirnaomics, Inc. | 6-
Nov- 07 |
26-
May -16 |
29-
May -18 |
| USRE46630 | Substituted 4-pyridones and their use as inhibitors of neutrophil elastase activity | Boehringer Ingelhelheim International GmbH | 23-
Aug- 12 |
18-
Sep -15 |
12-
Dec -17 |
| USRE46441 | Circulation of components during homogenization of emulsions | NOVARTIS AG | 3-
Dec- 09 |
13-
Oct -15 |
20-
Jun- 17 |
| USH2284 | Vaccines for protecting against influenza | Novartis AG | 27-
Apr- 09 |
27-
Apr -10 |
3-
Sep -13 |
| USH2283 | Vaccines for protecting against influenza | Novartis AG | 27-
Apr- 09 |
27-
Apr -10 |
3-
Sep -13 |
| EP2517720A
1 |
Stabilized therapeutic small helical antiviral peptides | New York Blood Center, Inc. | 2-
Oct- 07 |
31-
Oct -12 |
31-
Oct- 12 |
| EP2510946A
1 |
Conjugates of synthetic tlr agonists and uses therefor | The Regents of The University of California | 7-
Feb- 08 |
17-
Oct -12 |
17-
Oct- 12 |
| EP2471938A
2 |
Recombinant polyvalent vaccine | National Institute of Biomedical Innovation | 24-
Nov- 05 |
24-
Nov -05 |
4-
Jul- 12 |
| EP2471937A
2 |
Recombinant polyvalent vaccine | National Institute of Biomedical Innovation | 24-
Nov- 05 |
24-
Nov -05 |
4-
Jul- 12 |
| EP2471936A
2 |
Recombinant polyvalent vaccine | National Institute of Biomedical Innovation | 24-
Nov- 05 |
24-
Nov -05 |
4-
Jul- 12 |
| EP2471551A
2 |
Decreasing potential iatrogenic risks associated with influenza vaccines | Novartis Vaccines and Diagnostics GmbH | 9-
Sep- 05 |
4-
Jul- 12 |
4-
Jul- 12 |
| EP2167534B
1 |
BIOACTIVE PEPTIDES AND METHOD OF USING SAME | Compugen Ltd. | 11-
Jul-08 |
4-
Jul- 12 |
4-
Jul- 12 |
| EP2121732B
1 |
COILED-COIL LIPOPEPTIDE HELICAL BUNDLES AND SYNTHETIC VIRUS-LIKE PARTICLES | UniversitÄat ZÄ’Arich Prorektorat Forschung | 6-
Dec- 07 |
4-
Jul- 12 |
4-
Jul- 12 |